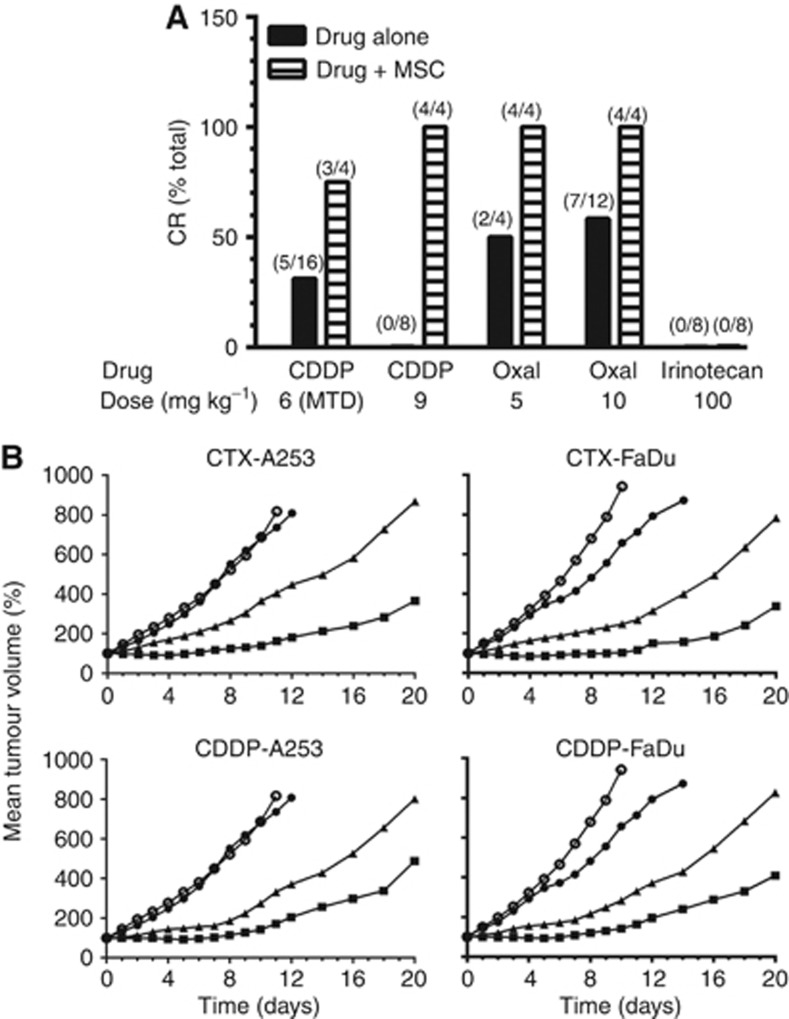
Figure 5.
Antitumour activity of CDDP, oxaliplatin, and irinotecan alone and in combination with MSC in rats bearing advanced Ward colorectal carcinoma (A) and CTX and CDDP alone and in combination with MSC in nude mice bearing advanced human A253 and FaDu squamous cell carcinoma xenografts (B). (A) CDDP and oxaliplatin (OXAL) were administered by a single i.v. injection and irinotecan by weekly for 4 weeks. Se-methylselenocysteine at 0.75 mg per rat per day p.o. daily for 21 days, with the first dose having started 14 days before CDDP or oxaliplatin treatment. For irinotecan group (16 rats) , MSC was administered daily for 14 days before and during irinotecan treatment for a total of 35 days. All treatments were initiated 14 days after tumour transplantation when the tumours reached ∼2500–3000 mg. In the oxaliplatin-alone (10 mg kg−1) group, 12 rats were evaluated, and in all other treatment groups, 4 rats were used. (B) ○ Untreated control; ● MSC 0.2 mg per mouse per day × 14; ▴ CTX 100 mg kg−1 or CDDP 8 mg kg−1, i.v. × 1; ▪ CTX 100 mg kg−1 or CDDP 8 mg kg−1 (i.v. × 1) + MSC (0.2 mg per mouse per day × 14). The treatment of CTX and CDDP was initiated on day 0 (7 days after tumour transplantation when the tumours reached ∼200–220 mg) and MSC by p.o. 7 days before and 7 days after CTX or CDDP in a total of 14 days. The mice were humanely killed when tumours reached ∼2000 mg. The numbers in parenthesis indicate the number of rats that achieved CR over the total number of rats treated.