Abstract
Abnormal intracellular Ca2+ cycling plays an important role in cardiac dysfunction and ventricular arrhythmias in the setting of heart failure and transient cardiac ischemia followed by reperfusion (I/R). We hypothesized that overexpression of the sarcoplasmic reticulum Ca2+ ATPase pump (SERCA2a) may improve both contractile dysfunction and ventricular arrhythmias. Continuous ECG recordings were obtained in 46 conscious rats after adenoviral gene transfer of either SERCA2a or the reporter gene β-galactosidase (βgal) or parvalbumin (PV), as early as 48 h before and 48 h after 30 min ligation of the left anterior descending artery by using an implantable telemetry system. Sham-operated animals were used for comparison for hemodynamic measurements, whereas within-animal baseline was used for electrocardiographic and echocardiographic parameters. All episodes of nonsustained ventricular tachycardia (VT) and ventricular fibrillation (VF) were counted, and their durations were summed by telemetry. I/R decreased regional cardiac wall thickening as well as the maximal rate of left ventricular pressure rise (+dP/dt) and ventricular pressure fall (–dP/dt). SERCA2a restored regional wall thickening and +dP/dt and –dP/dt to levels seen preoperatively. Regional-wall motion and anterior-wall thickening were improved in the SERCA2a animals, as assessed by echocardiography and piezoelectric crystals. To assess whether these effects are SERCA2a specific, we overexpressed a skeletal-muscle protein, PV, to examine whether Ca2+ buffering alone can mitigate ventricular arrhythmias. During the first hour after I/R, the rate of nonsustained VT plus VF was 16 ± 5 episodes per h (n = 6) in the Ad.βgal group, 22 ± 6 in the Ad.PV group, and 4 ± 2(n = 6, P < 0.01) in the Ad.SERCA2a group. The decrease in VT plus VF in the Ad.SERCA2a group was consistent throughout the 48 h of monitoring. These results show that improving intracellular Ca2+ handling by overexpression of SERCA2a restores contractile function and reduces ventricular arrhythmias during I/R.
Targeted gene transfer to diseased myocardium has resulted in improvement of ventricular function. These targets included the sarcoplasmic reticulum (SR) Ca2+AT Pase pump (SERCA2a), survival pathways such as akt, and the β2 receptors, among others (1). Over the years, experience with pharmacotherapy has shown that agents that enhance ventricular inotropy in diseased myocardium increase morbidity in terms of decreased survival and increased ventricular arrhythmias (2, 3). We have shown (4, 5) that gene transfer of SERCA2a is associated with improved ventricular function in an experimental model of heart failure. Unlike pharmacological inotropic agents, however, SERCA2a overexpression was associated with improved survival and enhanced energetic state (4, 5). An important yet answered issue regarding SERCA2a overexpression is whether increasing SR Ca2+ load would lead to oscillatory Ca2+ release from the SR and worsening arrhythmias. To induce Ca2+ overload acutely, we used a model of ischemia followed by reperfusion (I/R) in the rat. I/R in the rat has been studied extensively, and various forms of ventricular arrhythmias [extra beats, ventricular tachycardia (VT), and self-sustained ventricular fibrillation (VF)] have all been documented in this model by continuous telemetry (6). We investigated the role of SERCA2a and Ca2+ homeostasis in a model of I/R in rats. We overexpressed SERCA2a in rat hearts and subjected them to I/R while continuously monitoring their cardiac rhythm by implantable electrocardiographs. To verify that the effects in I/R are specifically due to SERCA2a overexpression and not just better buffering, we overexpressed PV also, which is a small intracellular, soluble binding protein found exclusively in fast-twitch muscle fibers having an affinity that is intermediate between troponin and SERCA, allowing it to act as a sink. This study allowed us to examine the effects of SERCA2a specifically on arrhythmias in a well documented model of Ca2+ overload.
Methods
Adenoviral Vectors. Recombinant adenoviral vectors were used with cytomegalovirus-driven expression cassettes for SERCA2a (Ad.SERCA2a), parvalbumin (Ad.PV), or β-galactosidase (Ad.βgal) with a second cassette in each adenovirus containing GFP substituted for E1 by means of homologous recombination (7). Ad.SERCA2a, Ad.PV, and Ad.βgal had concentrations of 12.0 × 1011, 7.0 × 1011, and 8.0 × 1010 pfu/ml, respectively, with a particle/pfu ratio of 50:1 and 40:1, respectively. Wild-type adenovirus contamination was excluded by the absence of PCR-detectable E1 sequences.
In Vitro Cardiomyocyte Hypoxia Model. Cardiomyocytes were prepared from 1- to 2-day-old rats, plated on glass coverslip, infected with or without Ad.SERCA2a and subjected to transient hypoxia for up to 24 h, as described (8). Contraction amplitude, rates of contraction and relaxation, and intracellular calcium transients were recorded online with an edge-detection system and data acquisition (IonOptix, Milton, MA) and analyzed after loading with fura-2 (Molecular Probes), as described (8), during hypoxia, immediately after reoxygenation, and 2 h after reoxygenation.
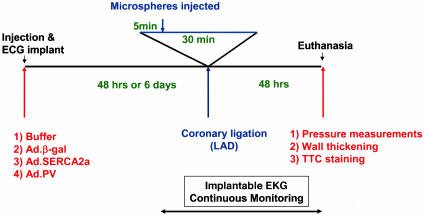
Animal Model and Quantification of Arrhythmias. The overall protocol for this study is shown in Fig. 1. Male 250- to 300-g Sprague–Dawley rats were anesthetized with 60 mg/kg pentobarbital i.p., intubated, and ventilated (SAR-830; CWE, Ardmore, PA). Two subsets of animals were studied. All animals underwent the following protocols. We injected 200 μl of buffer containing either Ad.SERCA2a, Ad.PV, or Ad.βgal by central thoracotomy into the anteroapical myocardium for 2–6 days before the EKG telemetry implantation and ischemic/reperfusion injury. This 2- to 6-day window constitutes the optimal time for gene expression. The telemetry device was implanted by using a hermetically sealed transmitter (7 g, 3 cm3) with a pair of helically wound, flexible stainless-steel wires (diameter, 0.6 mm) insulated with silicone tubing, except for the distal 1–2 cm implanted s.c. (Datasciences, Minneapolis) (Fig. 2). The transmitter was secured in the abdominal region, and the leads were tunneled under the skin to the recording sites and attached to the underlying tissue to prevent migration. The positive electrode was placed in a Lead II position; the negative electrode was secured over the right scapula. The biopotential signal was digitized, amplified, and continuously emitted with a radiofrequency carrier. Each rat was then housed in an individual cage placed on a receiver that continuously captured the signal, independently of animal activity. After reconversion to analog format and filtering at 100 Hz, a continuous data stream was fed into a personal computer equipped with an analog-to-digital converter (AT-MIO-16X, National Instruments, Austin, TX). The data were digitized with 16-bit precision, processed continuously, and displayed in real-time with a sampling rate of 500 Hz. The data were simultaneously stored in a continuous binary data file for later analysis. To induce I/R, left thoracotomy was again performed, and the left anterior descending coronary artery (LAD) was ligated with 6-0 silk suture 4 mm from its origin with a slipknot. Ischemia was confirmed by myocardial blanching and EKG evidence of injury (Fig. 3). At 5 min into ischemia, 300 μl of fluorescent 10-μm FluoSphere microspheres (Molecular Probes) were injected into the left ventricular (LV) cavity. After 30 min, the LAD ligature was released and reperfusion was confirmed visually. For sham I/R injury, thoracotomy was performed without LAD ligation.
Fig. 1.
Schematic representation of the protocol used for the evaluation of the effect of SERCA2a overexpression on I/R injury and evaluated parameters. At 2–6 days before ischemia induction, animals in each group were injected either with buffer or adenovirus carrying the βgal, SERCA2a, or PV gene. After 2–6 days, the LAD was ligated for 30 min. At 5 min into the ligation, 400 μlof fluorescent mycrospheres were injected into the LV cavity. At 48 h after I/R, the rats underwent echocardiographic evaluation and hemodynamic measurements using piezoelectric crystals and a pressure catheter. The rats were then killed, and the heart of each animal was excised and sectioned for TTC staining.
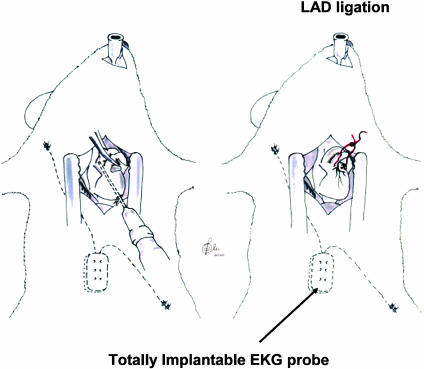
Fig. 2.
Drawing depicting the insertion of the implant of the EKG probe, the in vivo gene transfer, and the ligation of the LAD.
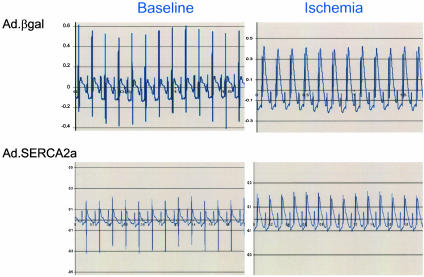
Fig. 3.
Representative EKG traces showing ST-segment elevation as index of ischemia induced by LAD ligation. An equivalent EKG signal of ischemia is shown in the reporter gene and in the SERCA2a-treated animals.
Hemodynamic Measurements. At 24 h after ischemia or sham operation, the first subset of rats underwent thoracotomy and placement of a 1.8F LV pressure transducer (Millar Instruments, Houston). Piezoelectric crystals (0.5-mm; Sonometrics, Ontario) were placed on the anterior epicardial and endocardial LV surfaces. Regional wall thickening (anterior epicardial to endocardial) was calculated from digitally acquired piezoelectric crystal position (Sonometrics). Pressure measurements were digitized at 1.0 kHz and analyzed with commercially available sonolab software (Sonometrics) to derive the maximal rates of ventricular pressure rise (+dP/dt) and ventricular pressure fall (–dP/dt).
Echocardiographic Evaluation. Before induction of ischemia and reperfusion, immediately after and 24 h later, wall motion and wall thickening was evaluated on M-mode echocardiography measured from a short-axis view at the level of the papillary muscles. A 128XP/10c (Acuson, Mountain View, CA) with a 13-Hz probe was used to perform the study.
Infarct Size. Rats were killed 48 h after ischemia. Hearts were sectioned from apex to base into four 1-mm sections by using a blade on a coronal-heart slicer matrix (Braintree Scientific). We used 1-mm sections to quantify the area at risk (AAR) and the infarct area. To delineate the infarct, sections were incubated in 1% (wt/vol) triphenyltetrazolium chloride (TTC; Sigma) in PBS (pH 7.4) at 32°C for 10 min. The territory of perfused vessels was indicated by the area perfused with red fluorescent microspheres. For each section, the AAR and infarct area were measured from enlarged digital micrographs with image software (National Institutes of Health, Bethesda). The AAR was defined by the area delineated by the absence of microspheres. Percentage of myocardial infarction was calculated as the total infarcted area, unstained by TTC, divided by the total AAR for that heart.
Statistical Analysis. Data are presented as mean ± SD. Data were compared by two-tailed t test or ANOVA, as appropriate, with statview (Abacus Concepts, Berkeley, CA). The null hypothesis was rejected for P < 0.05.
Results
Survival. In vivo gene transfer was performed on 46 animals. An overall survival of 80% was obtained in all groups of animals undergoing I/R. However, both early and late mortality was increased by 50% in the Ad.βgal and the Ad.PV-treated animals compared with SERCA2a on I/R injury.
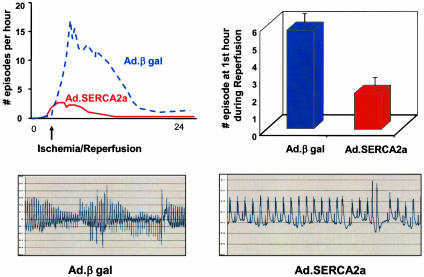
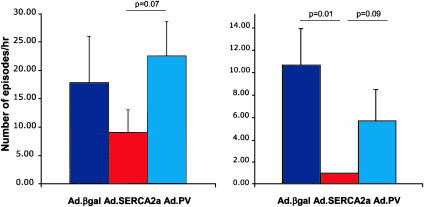
Electrocardiographic Data. Analyses of the baseline recordings before coronary artery occlusion revealed that VT or VF were mainly absent or exceptionally rare in rats without coronary occlusion. During and after coronary occlusion, however, all of the animals developed at least one episode of VT or VF. During ischemia, all groups showed significant ST-segment elevations, as shown in Fig. 3. As shown in Fig. 4, the number of episodes of VT and VF increased sharply during ischemia and remained elevated during reperfusion. Both VT and VF were present during this time period (in rats, VF can be self-limited and nonfatal). Most episodes of VT and VF terminated spontaneously, typically followed by a pause and later by a phase of slowly accelerating ventricular-escape rhythm in the presence of various degrees of atrioventricular-conduction block. Overexpression of SERCA2a significantly decreased the number of VT and VF episodes, as shown in Fig. 4. As shown in Fig. 5, the number of episodes during the 30 min of occlusion was significantly lower in SERCA2a-overexpressing rats, and similarly, the number of episodes during the first 30 min of reperfusion was significantly lower in SERCA2a-overexpressing rats, as shown in Fig. 5. Overexpression of PV did not offer any protection from VT or VF during coronary occlusion or reperfusion.
Fig. 4.
Time course for the incidence 24 h after I/R, showing a profound decrease of the incidence of arrhythmic episodes (VT and VF) per hour in the SERCA2a group compared with βgal as well as VT and VF on reperfusion. Representative traces are shown below the quantitative graphs.
Fig. 5.
Tabulated incidence of VT and VF during 30 min of ischemia and the subsequent 30 min of reperfusion in rats overexpressing Ad.GFP-βgal (n = 4), Ad.SERCA2a (n = 3), or Ad.PV (n = 6).
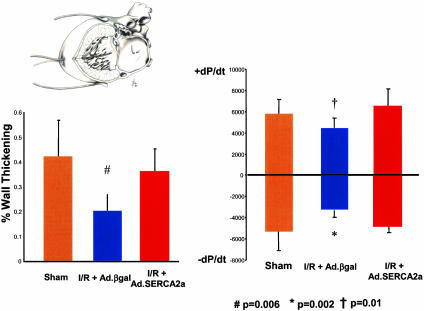
Hemodynamic Measurements. Cardiac function was analyzed at 48 h in a subgroup of animals. The maximal rates of +dP/dt and –dP/dt were reduced significantly by I/R in control Ad.βgal rats (Fig. 6). Of note, extensive previous physiological measurements have documented that control virus does not affect in vivo cardiac function. SERCA2a overexpression in I/R, however, significantly increased both +dP/dt and –dP/dt compared with the I/R plus Ad.βgal, restoring both to levels seen in sham-operated controls. We also evaluated cardiac-wall motion with piezoelectric crystals placed on the epicardial and endocardial surfaces of the LV anterior wall (Fig. 6 Left). I/R reduced systolic thickening in the ischemic region in I/R plus Ad.βgal, whereas SERCA2a overexpression preserved thickening at levels comparable with sham-operated animals (Fig. 6).
Fig. 6.
Inset shows the method of hemodynamic measurements, with the piezoelectric crystals placed across the anterior wall of the LV and the pressure probe inside the LV cavity, as described previously. To determine cardiac function in vivo, +dP/dt and –dP/dt were derived from measures of LV pressure 24 h after I/R or sham operation. Ad.SERCA2a prevented decrease in +dP/dt and –dP/dt seen in control animals after I/R. Anterior systolic thickening was measured by microsonometry with epicardial and endocardial piezoelectric crystals. Ad.SERCA2a expression preserved systolic thickening at levels comparable with sham-operated animals and significantly better than Ad.βgal transduced animals after I/R. (SERCA2a, n = 6; βgal, n = 8; Sham, n = 7.)
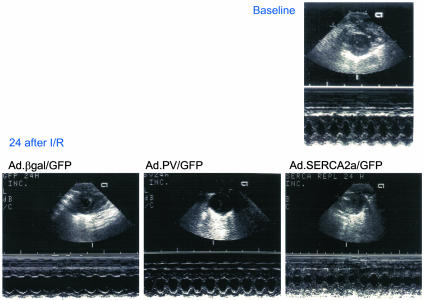
Echocardiographic Measurements. Echocardiographic measurement of anterior wall motion and thickness from short-axis views at the level of the papillary muscle revealed a reduced wall motion in the βgal- and PV-treated animals and a preserved function in the SERCA-overexpressing rats (Fig. 7). A 15% and 13% reduction in anterior-wall thickening in systole was measured in the I/R plus Ad.βgal group and I/R plus Ad.PV groups, respectively, whereas the rat hearts overexpressing SERCA2a rats showed a 4% reduction. Fractional shortening was reduced by 21%, 31%, and 8% in the I/R plus Ad.PV, I/R plus Ad.βgal, and I/R plus Ad.SERCA2a rats, respectively. Table 1 shows wall-thickness values after I/R in the Ad.SERCA2a, Ad.βgal, and Ad.PV groups.
Fig. 7.
The wall motion and wall thickness were evaluated noninvasively before I/R and 48 h after I/R in the animal groups treated with Ad.βgal, Ad.SERCA2a, and Ad.PV. Upper shows the baseline wall movement in M-mode from Ad.βgal-, Ad.SERCA2a-, and Ad.PV-treated animals. Lower shows the corresponding parameters after I/R in the same groups. A protection from wall-motion abnormalities corresponding to the invasive measurements was observed in the SERCA2a-treated animals.
Table 1. Echocardiographic measurements after ischemia and reperfusion injury.
| Group | AW s | AW d | PW s | FS |
|---|---|---|---|---|
| SERCA (n = 7) | 2.57 ± 0.18 | 1.67 ± 0.15 | 2.41 ± 0.23 | 71.43 ± 3.68 |
| βgal (n = 5) | 1.82 ± 1.14* | 1.34 ± 0.10† | 2.42 ± 0.04 | 51.97 ± 9.2‡ |
| PV (n = 5) | 1.70 ± 0.06* | 1.40 ± 0.06 | 2.24 ± 0.19 | 52.10 ± 2.78§ |
AW, anterior wall; s, systole; d, diastole; PW, posterior wall; FS, fractional shortening.
P = 0.01, compared with SERCA2a.
P = 0.08, compared with SERCA2a.
P = 0.001, compared with SERCA2a.
P = 0.04, compared with SERCA2a.
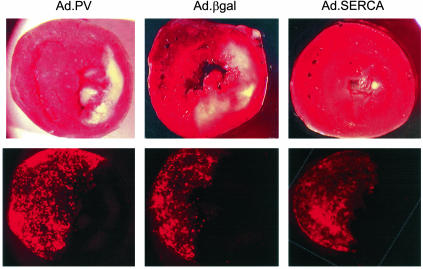
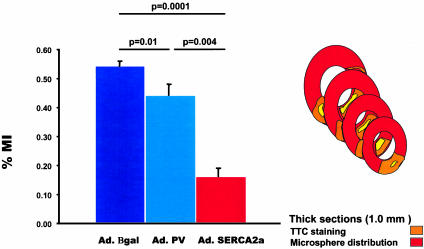
Myocardial Infarction After Ischemia Reperfusion. The ischemic area induced by LAD ligation (%AAR) did not differ among the three groups, as shown in Fig. 8, in which each representative section of each group shows the extent of infarct with respect to the AAR, as delineated by the distribution of fluorescent beads. A quantification of the average of all section measurements for each heart in the three groups is shown in Fig. 8. SERCA2a overexpression significantly reduced infarct size compared with the Ad.βgal-injected group (Fig. 9).
Fig. 8.
Sections of hearts stained with TTC immediately after rats were killed. Upper shows representative sections of TTC from hearts overexpressing Ad.βgal, Ad.SERCA2a, and Ad.PV. Lower shows the distribution of the fluorescent beads in the perfused regions.
Fig. 9.
Tabulated data from the TTC staining, showing a significant reduction in the percentage of myocardial infarction (%MI) after overexpression of Ad.SERCA2a compared with Ad.PV or Ad.βgal. Inset is a drawing depicting the method of measuring %MI. Red indicates the perfused area as delineated by the fluorescent beads. Orange indicates the AAR of vital myocardium as marked by the TTC staining. Yellow indicates the infracted area of dead myocardium. The reconstruction of the total MI results from the average measurements of consecutive cuts. (SERCA, n = 4; GFP, n = 4; Sham, n = 4.)
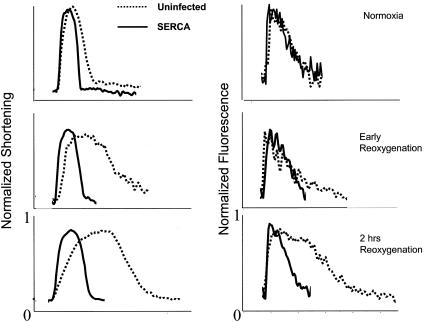
Cardiomyocyte Function in Vitro. Given the improvement in cardiac function in vivo, we examined the functional effects of SERCA2a overexpression to transient hypoxia and reoxygenation in vitro. In neonatal cardiomyocytes, hypoxia for 24 h followed by reoxygenation induces prolonged relaxation and impaired calcium uptake into the SR, as shown in Fig. 10. Expression of SERCA2a protected from hypoxia/reoxygenation-induced myocyte relaxation abnormality, preserving contractile function and calcium handling comparable to normoxic conditions at immediately after reoxygenation and 2 h after reoxygenation.
Fig. 10.
Cardiomyocyte function in vitro. After 24 h of hypoxia, Ad.SERCA2a protected from the prolongation of relaxation and the calcium transient immediately and 2 h after relaxation.
Discussion
Ventricular arrhythmias are common events often leading to sudden death in ischemic and nonischemic cardiomyopathy and heart failure (2, 3). We addressed the important issue of whether increasing Ca2+ load would lead to increased oscillatory Ca2+ sparks waves, which worsen arrhythmias and can be prevented by SERCA2a overexpression. To induce Ca2+ overload acutely, we used a rat model of I/R.
I/R of myocardium induces ventricular arrhythmias including ventricular premature beats, VT and VF in both experimental animal models and in man (9–11). Reperfusion of the ischemic myocardium results in an increase in intracellular Na+ and Ca2+ (9–11). The overload of these ions occurring in myocardial cells appears to be a very important etiologic factor in myocardial injuries (ischemia and heart failure) and arrhythmias.
With reduced ATP in the cell, many ATPase pumps, including SERCA2a, have decreased activity (12). This reduction in activity results in decreased SR Ca2+ load and elevated diastolic Ca2+ that induce after-depolarizations and triggered activity, culminating in ventricular arrhythmias (11). Triggered arrhythmias, including delayed after-depolarization (which occur when repolarization is completed and are mostly associated with cellular Ca2+ overload), and early after-depolarizations [which are secondary depolarizations occurring before completion of the action-potential (AP) repolarization] are major initiators of VT and VF and, thus, fatal sudden events (11).
Under normal conditions, the excitation to contraction coupling is regulated by the sequential changes of ionic currents involved in the balance and timing of the membrane potential and the AP in the specialized pacemaker cells and in the myocytes (11). Imbalance of timing and activation of these heterogeneous contributors to pacemaker activity lead to arrhythmogenic events. K+ channel currents during the AP contribute to the repolarization phase by outward K+ currents (Ito), as well as the inward rectifier K+ current (IK1), which is meant to stabilize the membrane resting potential (Em) (11). A reduction in K+ currents, as occur in failing hearts, leads to a more positive (depolarized) Em and to triggered activities (early after-depolarizations). The failing myocytes also show a smaller Ca2+ transients that may cause less complete inactivation of the inward Ca2+ current (ICa) (physiological trigger of SR Ca2+ release) during the early phases of the AP. Those factors combine to increase the likelihood of reactivation of inward ICa and early after-depolarizations (11).
In addition to the reduced K+ currents and SERCA2a function, Na+–Ca2+ exchange is increased in failing hearts (13). This increase in Na+–Ca2+ exchange activity means that a given SR Ca2+ release will produce more activated inward transient current (Iti) and, thus, more inward Na/Ca current, leading to more delayed after-depolarizations. Further, any given Iti will produce a greater delayed after-depolarization because there is less IK1 to stabilize resting-membrane potential. Thus, only half as much SR Ca2+ release is required to cause a delayed after-depolarization that reaches the threshold to trigger an arrhythmogenic AP.
In addition, during ischemia, protons (H+) leaking from damaged cells, accumulate in the extracellular space, and the rapid washout of extracellular H+ during reperfusion may create an intracellular–extracellular H+ gradient, resulting in an influx of Na+ by the Na+–H+ exchanger (14). Such rise in intracellular Na+ concentration by the Na+–H+ exchange system, in turn, increases the intracellular Ca2+ concentration by means of the Na+–Ca2+ exchange. This Ca2+ increase, leading to Ca2+ overload, induces arrhythmias and cell death.
In our study, we found that SERCA2a overexpression significantly decreased ventricular arrhythmias during I/R and 24 h later. In addition, SERCA2a overexpression significantly reduced infarct size and improved wall thickening in the anterior wall. The decrease in infarct size may have contributed to the decrease in ventricular arrhythmias because the size of the infarct is associated with the incidence and frequency of arrhythmias. Furthermore, this large decrease in infarct size is most likely due to the survival of cardiomyocytes in the AAR after I/R. A decrease in diastolic Ca2+ and better handling of intracellular ions during the rush of reperfusion are both associated with improved survival of the cardiomyocyte. The reduced incidence and severity of threatening arrhythmias on I/R in the SERCA2a-overexpressing animals as compared with I/R plus Ad.βgal also correspond to a preservation of muscle function, as shown by improved wall thickening and hemodynamic measurements. To determine whether these effects are specific to SERCA2a or are simply an effect of better buffering, we compared the effects of overexpressing SERCA2a to the effects of overexpressing PV. PV is small (11 kDa) intracellular, soluble Ca2+ binding protein found exclusively in fast-twitch muscle fibers. It has a calcium affinity that is intermediate between troponin and SERCA, allowing it to act as a Ca2+ sink to temporarily bind Ca2+ before SR uptake. PV gene transfer is one approach that has recently been used successfully to target prolonged relaxation in a rodent model of diastolic dysfunction in short term (15). Adenoviral transduction of PV into the LV free wall has been shown to accelerate LV isovolumic relaxation times significantly in normal animals and also to restore normal relaxation performance in hypothyroidism-induced diastolic dysfunction (15). However, overexpression of PV in our model did not mitigate the effects of I/R on contractile dysfunction in the anterior wall, as evidenced by the echocardiographic data, and did not decrease the incidence of arrhythmias. The abrogation of ventricular arrhythmias, therefore, can be specific to SERCA2a overexpression, and this difference may be due to the fact that simply buffering calcium is not sufficient to decrease triggered activity. Furthermore, the large amount of calcium influx during I/R may overwhelm the buffering capacity of PV. Giving that there are multiple ways in which Ca2+ interplays with other ions in modulating AP and cardiac rhythm, the interplay between SERCA2a and other transporters along with the loading of SR with Ca2+ is an important characteristic of the cardioprotective effect of SERCA2a.
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL-069842 (to F.d.M.), HL-057623, and HL-071763 (to R.J.H.). R.J.H. is an American Federation of Aging Research Paul Beeson Scholar.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: I/R, ischemia followed by reperfusion; SR, sarcoplasmic reticulum; SERCA2a, SR Ca2+ ATPase pump; PV, parvalbumin; βgal, β-galactosidase; VT, ventricular tachycardia; VF, ventricular fibrillation; +dP/dt, ventricular pressure rise; –dP/dt, ventricular pressure fall; LAD, left anterior descending coronary artery; LV, left ventricular; AAR, area at risk; TTC, triphenyltetrazolium chloride; AP, action potential.
References
- 1.Hajjar, R. J., del Monte, F., Matsui, T. & Rosenzweig, A. (2000) Circ. Res. 86, 616–621. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson, L. W. (2003) Circulation 108, 492–427. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson, L. W. (2003) Circulation 108, 367–372. [DOI] [PubMed] [Google Scholar]
- 4.del Monte, F., Harding, S. E., Schmidt, U., Matsui, T., Kang, Z. B., Dec, G. W., Gwathmey, J. K., Rosenzweig, A. & Hajjar, R. J. (1999) Circulation 100, 2308–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Monte, F., Williams, E., Lebeche, D., Schmidt, U., Rosenzweig, A., Gwathmey, J. K., Lewandowski, D. E. & Hajjar, R. J. (2001) Circulation 104, 1424–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Opitz, C. F., Mitchell, G. F., Pfeffer, M. A. & Pfeffer, J. M. (1995) Circulation 92, 253–261. [DOI] [PubMed] [Google Scholar]
- 7.He, T. C., Zhou, S., da Costa, L. T., Yu, J., Kinzler, K. W. & Vogelstein, B. (1998) Proc. Natl. Acad. Sci. USA 95, 2509–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsui, T., Tao, J., del Monte, F., Lee, K. H., Li, L., Picard, M., Force, T. L., Franke, T. F., Hajjar, R. J. & Rosenzweig, A. (2001) Circulation 104, 330–335. [DOI] [PubMed] [Google Scholar]
- 9.Bers, D. M. (2000) Circ. Res. 87, 275–281. [DOI] [PubMed] [Google Scholar]
- 10.Bers, D. M. (2002) Nature 415, 198–205. [DOI] [PubMed] [Google Scholar]
- 11.Bers, D. M. (2002) Circ. Res. 90, 14–17. [PubMed] [Google Scholar]
- 12.Overend, C. L., Eisner, D. A. & O'Neill, S. C. (2001) Circ. Res. 88, 181–187. [DOI] [PubMed] [Google Scholar]
- 13.Hasenfuss, G., Schillinger, W., Lehnart, S. E., Preuss, M., Pieske, B., Maier, L. S., Prestle, J., Minami, K. & Just, H. (1999) Circulation 99, 641–648. [DOI] [PubMed] [Google Scholar]
- 14.Lu, H. R., Yang, P., Remeysen, P., Saels, A., Dai, D. Z. & De Clerck, F. (1999) Eur. J. Pharmacol. 365, 233–239. [DOI] [PubMed] [Google Scholar]
- 15.Wahr, P. A., Michele, D. E. & Metzger, J. M. (1999) Proc. Natl. Acad. Sci. USA 96, 11982–11985. [DOI] [PMC free article] [PubMed] [Google Scholar]