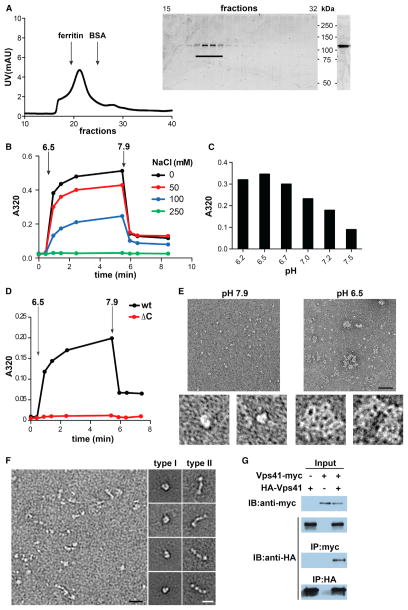
Figure 5. VPS41 Self-Assembles into a Lattice In Vitro.
(A) Full-length hVPS41 was purified from Sf9 cells by metal affinity chromatography followed by gel filtration (Superdex 200) in 10 mM Tris, pH 7.9 (left). Aliquots of the collected fractions were separated by electrophoresis through polyacrylamide (SDS-PAGE) and the proteins visualized by Coomassie staining (middle). Fractions containing monomeric hVPS41 were pooled, concentrated, and again visualized by Coomassie staining after SDS-PAGE (right).
(B–D) All self-assembly reactions were performed using ~10 μg protein in a total volume of 100 μl in the absence of NaCl unless otherwise indicated. Light-scattering measurements were taken before (t = 0) and after the addition of MES, pH 6.5 (B and D) or at the indicated pH (C). After 5 min, disassembly was triggered by the addition of Tris, pH 9.0. The bar graph (C) shows the values obtained after 5 min of assembly.
(E) Negative stain EM of recombinant hVPS41 shows single particles under conditions that do not permit assembly (left, pH 7.9) and irregular lattices (right) at low pH (6.5). The panels on the bottom show single particles (left) or a lattice (right) at higher magnification. Size bar represents 50 nm.
(F) Electron micrographs of unassembled hVPS41 (left panel) reveal two main types of particle: C-shaped (type I) and elongated (type II). Representative class averages for both particle types were computed from single particles sets and are shown on the right. Size bar represents 20 nm.
(G) COS7 cells were transfected with HA-VPS41 with or without VPS41-myc, as indicated. Cell lysates were immunoprecipitated with HA antibody and samples examined by immunoblotting with anti-myc or anti-HA antibodies (lower blots). Upper immunoblots show the expression of HA-VPS41 and VPS41-myc in the cell lysates.
See also Figure S3.