Abstract
Between November 2010, and May 2011, eleven cases of cholera, unrelated to a concurrent outbreak on the island of Hispaniola, were recorded, and the causative agent, Vibrio cholerae serogroup O75, was traced to oysters harvested from Apalachicola Bay, Florida. From the 11 diagnosed cases, eight isolates of V. cholerae were isolated and their genomes were sequenced. Genomic analysis demonstrated the presence of a suite of mobile elements previously shown to be involved in the disease process of cholera (ctxAB, VPI-1 and -2, and a VSP-II like variant) and a phylogenomic analysis showed the isolates to be sister taxa to toxigenic V. cholerae V51 serogroup O141, a clinical strain isolated 23 years earlier. Toxigenic V. cholerae O75 has been repeatedly isolated from clinical cases in the southeastern United States and toxigenic V. cholerae O141 isolates have been isolated globally from clinical cases over several decades. Comparative genomics, phenotypic analyses, and a Caenorhabditis elegans model of infection for the isolates were conducted. This analysis coupled with isolation data of V. cholerae O75 and O141 suggests these strains may represent an underappreciated clade of cholera-causing strains responsible for significant disease burden globally.
Introduction
Vibrio cholerae non-O1/non-O139 are the causative agents of sporadic, yet significant, gastrointestinal and extraintestinal infections globally, and it is well established that all strains of this species are capable of causing human infections that represent a significant global health burden [1], [2], [3], [4], [5], [6], [7]. Infection and subsequent illness caused by these organisms are linked to the presence of virulence factors in the core backbone of V. cholerae (hemolysins, lipases) or mobile pathogenicity islands (VPIs-1 and -2, and CTXΦ) that are frequently found in clinical isolates from cholera patients suffering severe rice water diarrhea [8], [9], [10]. Epidemic cholera is typically ascribed to V. cholerae serogroup O1 or O139; however, it is now understood that, similar to pathogenic Escherichia coli, a constellation of virulence factors along with host immune and nutritional status, are responsible for the severity and characteristic infections caused by these organisms [8], [9], [10], [11], [12]. It is established that those V. cholerae which acquire and express genes carried on mobile elements (O-antigens, VPI-1, VPI-2, CTXΦ, NAG-ST, etc.) are linked to epidemics of cholera. The scenario of mobile genetic element acquisition has been shown to have occurred within the 7th pandemic and PG-1 and -2 clades (12), but occurrence and persistence of such genetic constellations remains underappreciated in V. cholerae non-O1/non-O139 (non-PG) lineages. These elements, among many others, can be laterally transferred between strains of the same species or distantly related species in the environment [13], [14], [15] and give rise to virulent strains that potentially can cause epidemics. Further, these elements can be stable in V. cholerae non-O1/non-O139 isolates, as in strains of the 7th pandemic clade and persist in these conformations over time, ultimately conserved in the environment.
In developed nations, the leading cause of human disease caused by vibrios is consumption of raw or undercooked seafood, namely shellfish. In the United States, seafood-borne vibrioses have been traced to shellfish harvested from coastal (Atlantic and Pacific) regions, as far north as Alaska, but by far the majority of infections occur in the Gulf of Mexico, where the water temperature is warm, a parameter associated with increased Vibrio spp. densities as well as increased risk of vibriosis [16], [17], [18], [19], [20]. Recent cases of cholera traced to seafood consumption, and many V. parahaemolyticus infections and deaths caused by V. vulnificus have been reported in this region.
V. cholerae O75 serogroup strains have been reported to cause sporadic shellfish-borne cholera cases in the southeastern United States [21], [22]. Outbreaks caused by these strains are not continuous as outbreaks in developing nations because sanitation in the United States is such that untreated human waste is not typically discharged into water used for drinking, recreation, or harvesting of seafood and water used for consumption or for household use is typically treated to remove bacterial pathogens. Further, V. cholerae O75 strains have been isolated from environmental waters in the southeastern United States in the absence of reported cholera cases [21]. Here we present results of analysis of eight clinically recovered V. cholerae O75 isolates from an indigenous US Gulf Coast cholera outbreak that occurred in, 2010, and during March and April, 2011 [22].
Materials and Methods
Clinical V. cholerae isolates that were epidemiologically linked to consumption of oysters harvested from the Apalachicola Bay, FL were obtained from the Florida Department of Health Bureau of Public Health Laboratories in Jacksonville, FL. The genomes described in this study were either obtained from the NCBI Genbank database or, in the case of strains CP1110, 1111, 1112, 1113, 1114, 1115, 1116 and 1117, were sequenced using the Genome Analyzer IIx system (Illumina, Inc., San Diego, CA) according to the manufacturer's methods. Raw reads of these genomes were assembled with CLC Genomics Workbench. Genome-to-genome comparisons, identification and characterization of molecular genetic elements (MGEs), as well as core genome phylogenetics were performed by using methods described previously [12]. Genomes of V. cholerae strains CP1110 to CP1117 were annotated using Rapid Annotation using Subsystem Technology [23]. For in silico genomic island BLASTN and phylogenetic analyses the RAST-annotated ORFs of V. cholerae CP1110 were used as a reference. PCR analyses of virulence factors not resolved by genome sequencing (rstR alleles, nanH, and ctxB biotype) were done using the methods of Choi et al. [24], Vora et al. [25], and Nusrin et al. [26]. Phenotypic assays (proteolysis, hemolysis, biofilm formation, and motility) were conducted following methods standardized for V. cholerae [27]. Hemolysis, biofilm formation, motility, and proteolysis assays were done in nine replicates. BiOLOG phenotypic microarrays (PM1, PM2A, PM9, and PM10) were conducted in duplicate following the manufacturer's instructions (BiOLOG, Hayward, CA). Substrate metabolism was scored by dividing the area under the curve by the background values. Scores >2 were considered positive for metabolism of that substrate.
For the Caenorhabditis elegans model, SS104 glp-4 (bn2) temperature sensitive sterile strain was acquired from the Caenorhabditis Genetics Center (CGC). SS104 worms were maintained at 16°C, and experiments were performed at 25°C. Worms were cultured in C. elegans habitation media (CeHM) in tissue culture flasks on a platform shaker [28]. Adult nematodes were bleached (0.5 M NaOH, 1% Hypochlorite) to collect eggs, which were incubated in M9 media for 24 hours to bring them to synchronized L1 stage, and then transferred to CeHM. L4 stage worms were transferred to assay plates for survival experiments. Pathogen lawns for survival assays along with control bacteria E. coli OP50 were prepared by inoculating Nematode Growth Medium (NGM), in 6-cm Petri dishes, with 50 µl of an overnight V. cholerae culture. Plates were incubated overnight at room temperature before worms were added. Temperature sensitive sterile worms (SS104 glp-4(bn2)) strain, obtained from Caenorhabditis Genetics Center were transferred to NGM plates containing V. cholerae wild type strains E7946, CP1112, CP1114, CP1115 or E. coli OP 50 bacterial lawns and incubated at 25°C with ∼20–30 L4 stage worms added to each plate. Animals were scored every 24 h for survival. Animals were considered dead when they no longer responded to a gentle prod with a platinum wire. C. elegans survival was plotted using Kaplan-Meier survival curves and analyzed by log rank test using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). Survival curves resulting in p values of <0.05 relative to control were considered significantly different [29]. Strains and genomes used in this study are listed in Table 1.
Table 1. Genomes/strains used in this study.
| Organism | Strain ID | Serogroup/Serotype | Biotype | Geographical origin | Source of isolation | Year of isolation | Accession nos. |
| Vibrio cholerae | NCTC 8457 | O1 | El Tor | Saudi Arabia | Clinical | 1910 | NZ_AAWD01000000 |
| Vibrio cholerae | M66-2 | O1 | El Tor | Makassar, Indonesia | Clinical | 1937 | NC_012578/NC_012580 |
| Vibrio cholerae | MAK757 | O1 | El Tor | Sulawesi, Indonesia (Celebes Islands) | Clinical | 1937 | NZ_AAUS00000000 |
| Vibrio cholerae | O395 | O1 | Classical | India | Clinical | 1965 | NC_009456/NC_009457 |
| Vibrio cholerae | V52 | O37 | Sudan | Clinical | 1968 | NZ_AAKJ02000000 | |
| Vibrio cholerae | N16961 | O1 | El Tor | Bangladesh | Clinical | 1975 | NC_002505/NC_002506 |
| Vibrio cholerae | E7946 | O1 | El Tor | Bahrain | Clinical | 1978 | Not Sequenced |
| Vibrio cholerae | 2740-80 | O1 | El Tor | Gulf Coast, USA | Water | 1980 | NZ_AAUT01000000 |
| Vibrio cholerae | TM 11079-80 | O1 | El Tor | Brazil | Sewage | 1980 | NZ_ACHW00000000 |
| Vibrio cholerae | CT 5369-93 | O1 | El Tor | Brazil | Sewage | 1980 | NZ_ADAL00000000 |
| Vibrio cholerae | TMA21 | non-O1/non-O139 | Brazil | Water | 1982 | NZ_ACHY00000000 | |
| Vibrio cholerae | 12129(1) | O1 | El Tor | Australia | Water | 1985 | NZ_ACFQ00000000 |
| Vibrio cholerae | RC9 | O1 | El Tor | Kenya | Clinical | 1985 | NZ_ACHX00000000 |
| Vibrio cholerae | BX 330286 | O1 | El Tor | Australia | Water | 1986 | NZ_ACIA00000000 |
| Vibrio cholerae | V51 | O141 | USA | Clinical | 1987 | NZ_AAKI02000000 | |
| Vibrio cholerae | RC27 | O1 | Classical | Indonesia | Clinical | 1991 | NZ_ADAI00000000 |
| Vibrio cholerae | INDRE 91/1 | O1 | El Tor | Mexico | Clinical | 1991 | NZ_ADAK00000000 |
| Vibrio cholerae | C6706 | O1 | El Tor | Peru | Clinical | 1991 | NZ_AHGQ00000000 |
| Vibrio cholerae | CP1032(5) | O1 | El Tor | Mexico | Clinical | 1991 | NZ_ALDA00000000 |
| Vibrio cholerae | MO10 | O139 | Madras,India | Clinical | 1992 | NZ_AAKF00000000 | |
| Vibrio cholerae | Amazonia | O1 | Amazonia | Amazonas, Brazil | Clinical | 1992 | NZ_AFSV00000000 |
| Vibrio cholerae | MJ-1236 | O1 | El Tor | Matlab, Bangladesh | Clinical | 1994 | NC_012668/NC_012667 |
| Vibrio cholerae | IEC224 | O1 | El Tor | Belém, Brazil | Clinical | 1994 | NC_016944/NC_016945 |
| Vibrio cholerae | 1587 | O12 | Lima, Peru | Clinical | 1994 | NZ_AAUR01000000 | |
| Vibrio cholerae | RC385 | O135 | Chesapeake Bay, USA | Plankton | 1998 | NZ_AAKH02000000 | |
| Vibrio cholerae | CP1033(6) | O1 | El Tor | Mexico | Clinical | 2000 | NZ_AJRL00000000 |
| Vibrio cholerae | AM-19226 | O39 | Bangladesh | Clinical | 2001 | NZ_AATY01000000 | |
| Vibrio cholerae | MZO-3 | O37 | Bangladesh | Clinical | 2001 | NZ_AAUU01000000 | |
| Vibrio cholerae | MZO-2 | O14 | Bangladesh | Clinical | 2001 | NZ_AAWF01000000 | |
| Vibrio cholerae | 623-39 | non-O1/non-O139 | Bangladesh | Water | 2002 | NZ_AAWG00000000 | |
| Vibrio cholerae | CIRS101 | O1 | altered El Tor | Dhaka, Bangladesh | Clinical | 2002 | NZ_ACVW00000000 |
| Vibrio cholerae | CP1038 | O1 | El Tor | Zimbabwe | Clinical | 2003 | NZ_ALDC00000000 |
| Vibrio cholerae | B33 | O1 | El Tor | Beira, Mozambique | Clinical | 2004 | NZ_ACHZ00000000 |
| Vibrio cholerae | CP1040(13) | O1 | El Tor | Zambia | Clinical | 2004 | NZ_ALDD00000000 |
| Vibrio cholerae | CP1041(14) | O1 | El Tor | Zambia | Clinical | 2004 | NZ_ALDE00000000 |
| Vibrio cholerae | VC35 | O1 | El Tor | Kedah, Malaysia | Clinical | 2004 | NZ_AMBR00000000 |
| Vibrio cholerae | 3500-05 | O1 | El Tor | India | Clinical | 2005 | NZ_AHGL00000000 |
| Vibrio cholerae | 3582-05 | O1 | El Tor | Pakistan | Clinical | 2005 | NZ_AHGP00000000 |
| Vibrio cholerae | 3546-06 | O1 | El Tor | India | Clinical | 2006 | NZ_AHGM00000000 |
| Vibrio cholerae | LMA3984-4 | O1 | El Tor | Belém, Brazil | River Water | 2007 | CP002555/CP002556 |
| Vibrio cholerae | 3554-08 | O1 | El Tor | Nepal | Clinical | 2008 | NZ_AHGN00000000 |
| Vibrio cholerae | 3569-08 | O1 | El Tor | Gulf Coast, USA | Environmental | 2008 | NZ_AHGO00000000 |
| Vibrio cholerae | 2009V-1046 | O1 | El Tor | Pakistan | Clinical | 2009 | NZ_AHFX01000000 |
| Vibrio cholerae | 2009V-1085 | O1 | El Tor | Sri Lanka/India | Clinical | 2009 | NZ_AHFY00000000 |
| Vibrio cholerae | 2009V-1096 | O1 | El Tor | India | Clinical | 2009 | NZ_AHFZ00000000 |
| Vibrio cholerae | 2009V-1116 | O1 | El Tor | Pakistan | Clinical | 2009 | NZ_AHGA00000000 |
| Vibrio cholerae | 2009V-1131 | O1 | El Tor | India | Clinical | 2009 | NZ_AHGB00000000 |
| Vibrio cholerae | 2010V-1014 | O1 | El Tor | Pakistan | Clinical | 2009 | NZ_AHGG00000000 |
| Vibrio cholerae | 2011EL-1137 | O1 | El Tor | Zimbabwe | Clinical | 2009 | NZ_AHGJ00000000 |
| Vibrio cholerae | CP1048(21) | O1 | El Tor | Bangladesh | Clinical | 2010 | NZ_ALDJ00000000 |
| Vibrio cholerae | CP1050(23) | O1 | El Tor | Bangladesh | Clinical | 2010 | NZ_ALDK00000000 |
| Vibrio cholerae | EL1786 | O1 | El Tor | Haiti | Clinical | 2010 | NC_016445/NC_016446 |
| Vibrio cholerae | EL1798 | O1 | El Tor | Haiti | Clinical | 2010 | NZ_AELI00000000 |
| Vibrio cholerae | EL1792 | O1 | El Tor | Haiti | Clinical | 2010 | NZ_AELJ00000000 |
| Vibrio cholerae | HE-09 | non-O1/non-O139 | Haiti | Environmental | 2010 | NZ_AFOP00000000 | |
| Vibrio cholerae | HE-39 | non-O1/non-O139 | Haiti | Environmental | 2010 | NZ_AFOQ00000000 | |
| Vibrio cholerae | HE-48 | non-O1/non-O139 | Haiti | Environmental | 2010 | NZ_AFOR00000000 | |
| Vibrio cholerae | HC-02A1 | non-O1/non-O139 | Haiti | Clinical | 2010 | NZ_AFOT00000000 | |
| Vibrio cholerae | HC-21A1 | O1 | El Tor | Saint-Marc, Haiti | Clinical | 2010 | NZ_AGUK00000000 |
| Vibrio cholerae | HC-22A1 | O1 | El Tor | Saint-Marc, Haiti | Clinical | 2010 | NZ_AGUL00000000 |
| Vibrio cholerae | HC-32A1 | O1 | El Tor | Port-au-Prince, Haiti | Clinical | 2010 | NZ_AGUO00000000 |
| Vibrio cholerae | HC-72A2 | O1 | El Tor | Arcahaie, Haiti | Clinical | 2010 | NZ_AGUY00000000 |
| Vibrio cholerae | HC-80A1 | O1 | El Tor | Port-au-Prince, Haiti | Clinical | 2010 | NZ_AGVB00000000 |
| Vibrio cholerae | 2010EL-1961 | O1 | El Tor | Haiti | Clinical | 2010 | NZ_AHGD00000000 |
| Vibrio cholerae | 2010EL-2010H | O1 | El Tor | Haiti | Clinical | 2010 | NZ_AHGE00000000 |
| Vibrio cholerae | 2010EL-2010N | O1 | El Tor | Haiti | Clinical | 2010 | NZ_AHGF00000000 |
| Vibrio cholerae | 2011EL-1089 | O1 | El Tor | Haiti | Clinical | 2010 | NZ_AHGH00000000 |
| Vibrio cholerae | HC-41B1 | non-O1/non-O139 | Haiti | Clinical | 2010 | NZ_AJRP00000000 | |
| Vibrio cholerae | HE-40 | non-O1/non-O139 | Haiti | Hospital Latrine | 2010 | NZ_AJRX00000000 | |
| Vibrio cholerae | HE-46 | non-O1/non-O139 | Haiti | Gray Water | 2010 | NZ_AJRY00000000 | |
| Vibrio cholerae | HC-44C1 | non-O1/non-O139 | Haiti | Clinical | 2010 | NZ_AJSK00000000 | |
| Vibrio cholerae | HC-39A1 | non-O1/non-O139 | Haiti | Clinical | 2010 | NZ_ALDM00000000 | |
| Vibrio cholerae | HC-41A1 | non-O1/non-O139 | Haiti | Clinical | 2010 | NZ_ALDN00000000 | |
| Vibrio cholerae | HC-42A1 | non-O1/non-O139 | Haiti | Clinical | 2010 | NZ_ALDO00000000 | |
| Vibrio cholerae | HC-47A1 | non-O1/non-O139 | Haiti | Clinical | 2010 | NZ_ALDR00000000 | |
| Vibrio cholerae | HE-16 | non-O1/non-O139 | Haiti | Gray Water | 2010 | NZ_ALEB00000000 | |
| Vibrio cholerae | HE-25 | non-O1/non-O139 | Haiti | Gray Water | 2010 | NZ_ALEC00000000 | |
| Vibrio cholerae | HE-45 | non-O1/non-O139 | Haiti | Gray Water | 2010 | NZ_ALED00000000 | |
| Vibrio cholerae | CP1042(15) | O1 | El Tor | Thailand | Clinical | 2010 | NZ_ALDF00000000 |
| Vibrio cholerae | BJGO1 | non-O1/non-O139 | Mississippi Gulf Coast, USA | Clinical | 2010 | NZ_AFOU00000000 | |
| Vibrio cholerae | 2010EL-1749 | O1 | El Tor | western Africa | Clinical | 2010 | NZ_AHGC00000000 |
| Vibrio cholerae | 2011EL-1133 | O1 | El Tor | Haiti | Clinical | 2011 | NZ_AHGI00000000 |
| Vibrio cholerae | 2011V-1021 | O1 | El Tor | Dominican Republic | Clinical | 2011 | NZ_AHGK00000000 |
| Vibrio cholerae | 2011EL-301 | non-O1/non-O139 | Taganrog, Russia | Water | 2011 | NZ_AJFN00000000 | |
| Vibrio cholerae | CP1110 | O75 | Florida Gulf Coast, USA | Clinical | 2010–2011 | NZ_AMWF00000000 | |
| Vibrio cholerae | CP1111 | O75 | Florida Gulf Coast, USA | Clinical | 2010–2011 | NZ_AMWS00000000 | |
| Vibrio cholerae | CP1112 | O75 | Florida Gulf Coast, USA | Clinical | 2010–2011 | NZ_AMWT00000000 | |
| Vibrio cholerae | CP1113 | O75 | Florida Gulf Coast, USA | Clinical | 2010–2011 | NZ_AMWU00000000 | |
| Vibrio cholerae | CP1114 | O75 | Florida Gulf Coast, USA | Clinical | 2010–2011 | NZ_AMWV00000000 | |
| Vibrio cholerae | CP1115 | O75 | Florida Gulf Coast, USA | Clinical | 2010–2011 | NZ_AMWR00000000 | |
| Vibrio cholerae | CP1116 | O75 | Florida Gulf Coast, USA | Clinical | 2010–2011 | NZ_ANNM00000000 | |
| Vibrio cholerae | CP1117 | O75 | Florida Gulf Coast, USA | Clinical | 2010–2011 | NZ_AMWW00000000 | |
| Vibrio cholerae | VL426 | non-O1/non-O139 | albensis | Maidstone, Kent, UK | Water | NZ_ACHV00000000 | |
| Vibrio anguillarum | 96F | O1 | Chesapeake Bay, USA | Striped bass (Morone saxatilis) | NZ_AEZA00000000 | ||
| Vibrio anguillarum | RV22 | O2β | Atlantic coast of Spain | Turbot (Scophthalmus maximus) | NZ_AEZB00000000 | ||
| Vibrio anguillarum | 775 | O1 | coast of Washington state, USA | Coho salmon (Oncorhynchus kisutch) | NC_015633/NC_015637 | ||
| Vibrio coralliilyticus | ATCC BAA-450 | Zanzibar, Tanzania | Coral | 1999 | NZ_ACZN00000000 | ||
| Vibrio mimicus | SX-4 | China | Clinical | 2009 | NZ_ADOO00000000 | ||
| Vibrio mimicus | VM573 | United States | Clinical | 1990s | NZ_ACYV00000000 | ||
| Vibrio mimicus | MB-451 | Matlab, Bangladesh | Clinical | NZ_ADAF00000000 | |||
| Vibrio orientalis | CIP 102891 | Yellow sea, China | Water | NZ_ACZV00000000 | |||
| Vibrio parahaemolyticus | RIMD 2210633 | O3:K6 | Japan | Clinical | 1996 | NC_004603/NC_004605 | |
| Vibrio splendidus | 12B01 | Plum Island Estuary, Massachusetts, USA | Water | NZ_AAMR00000000 | |||
| Vibrio vulnificus | YJ016 | biotype 1 | Taiwan | Clinical | NC_005139/NC_005140 | ||
| Vibrio sp. RC341 | RC341 | O153 | Chesapeake Bay, USA | Water | 1998 | NZ_ACZT00000000 | |
| Grimontia hollisae | CIP 101886 | Maryland, USA | Clinical | NZ_ADAQ00000000 |
Results and Discussion
Phylogenomic Analysis of Florida Outbreak Strains
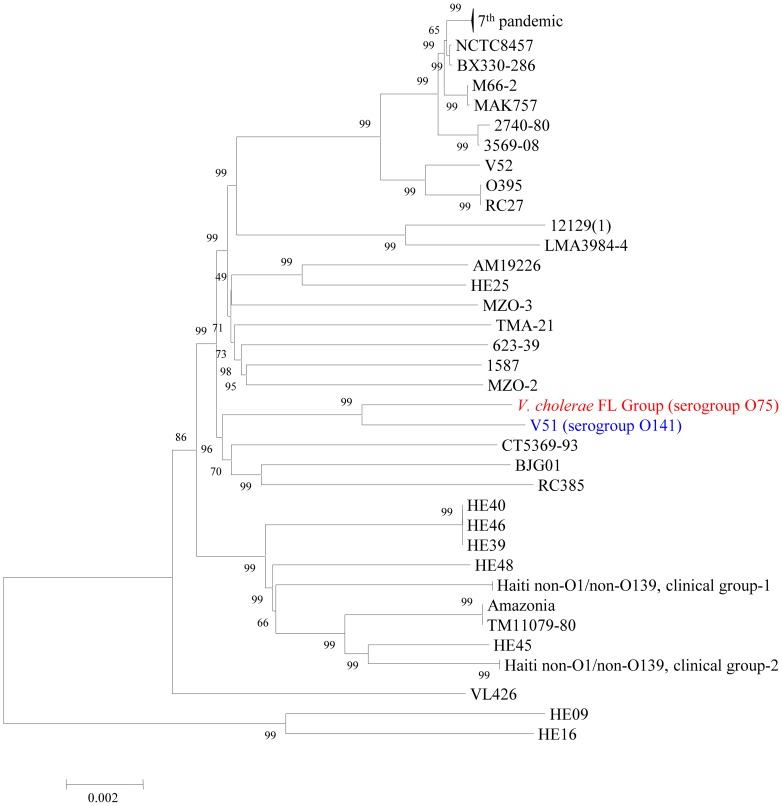
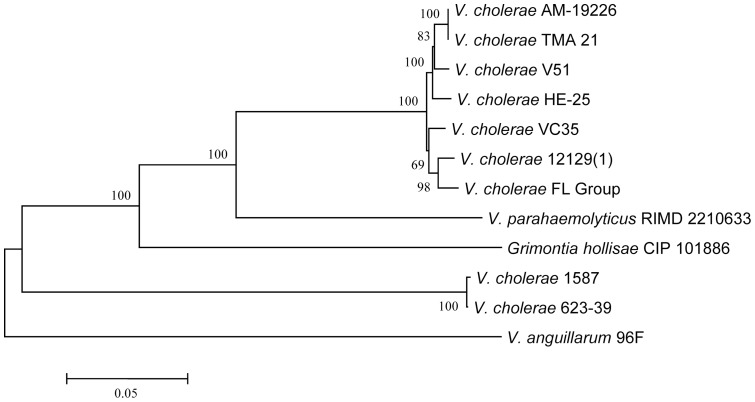
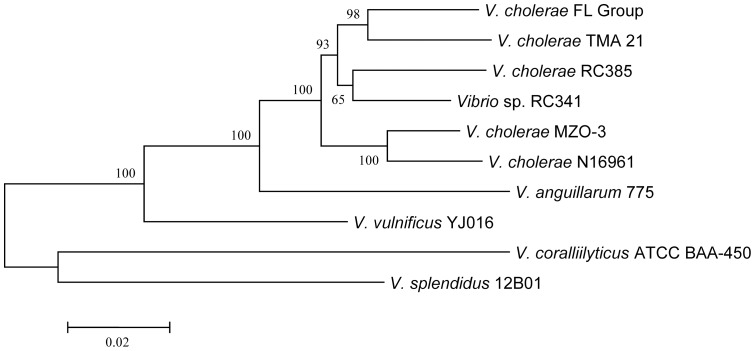
The eight isolates subjected to analysis in this study have been labeled by number (isolates CP1110, 1111, 1112, 1113, 1114, 1115, 1116 and 1117) and are hereafter collectively referred to as the V. cholerae FL Group. The phylogeny of 84 fully and partially sequenced V. cholerae strains, including the eight V. cholerae FL Group genomes, was inferred (Figure 1). Results of the analysis demonstrate that the V. cholerae FL Group are sister taxa with V. cholerae V51, a clinical V. cholerae O141 serogroup strain isolated from a human clinical case in the United States in 1987, suggesting a common ancestor after it had diverged from other V. cholerae lineages. From a public health perspective, the results of the analysis demonstrate the group represents a phyletic lineage of V. cholerae non-O1/non-O139 strains that persist in the United States as a cause of morbidity. Although, not added to this analysis due to the absence of their sequenced genomes, results of this analysis coupled with V. cholerae isolation data from cholera patients worldwide demonstrate that other V. cholerae serogroup O141 and O75 strains result in similar clinical manifestations as the strains in this study, that is symptoms of cholera [30], [31]. As with the isolates sequenced in this analysis, other V. cholerae O141 and O75 infections in the United States were associated with either seafood consumption or presence of the patient in a coastal state, suggesting infections with strains of these serogroups are transmitted to people in a similar manner as those of the O1 serogroup and therefore they have a similar ecology as serogroup O1 strains in the United States [32], [33].
Figure 1. Neighbour-joining tree inferring phylogenetic relationships of 84 V. cholerae genomes based on 995 orthologous protein-coding genes (954,646 bp).
V. cholerae FL Group is labelled in red and V. cholerae V51 is labelled in blue. Haiti non-O1/non-O139 clinical groups-1 and -2 are further defined by Hasan et al. [6]. Numbers at nodes represent bootstrap values. Nucleotide substitution model is the Kimura-2-parameter. Bar length = 0.002 nucleotide substitutions per site.
We identified 8 single nucleotide polymorphisms (SNPs) among the V. cholerae O75 genomes in this study. Six of these occurred in six separate ORFs and two occurred in one ORF annotated as a “putative transcriptional activator ToxR.” It is not clear if these SNPs influence the ecology or virulence potential of these isolates. However, they do demonstrate an appreciable level of genomic diversity between strains of the same outbreak (Table 2). To further estimate the genomic diversity of this lineage, comparisons should be made to other V. cholerae O75 isolates from clinical and environmental isolates.
Table 2. ORFs with polymorphisms within the V. cholerae FL group.
| N16961 Locus | CP1110 | CP1111 | CP1112 | CP1113 | CP1114 | CP1115 | CP1116 | CP1117 | Annotation |
| VC0315 | A | A | A | A | G | G | A | A | CDP-diacylglycerol–serine O-phosphatidyltransferase |
| VC1899 | C | C | C | C | T | C | C | C | hypothetical protein |
| VC0028 | C | C | C | C | C | C | T | C | Dihydroxy-acid dehydratase |
| VC0031 | C | C | C | C | C | C | C | A | Acetolactate synthase large subunit |
| VC1359 | T | T | T | T | C | C | T | T | ABC-type polar amino acid transport system ATPase component |
| GI-26* | G | G | T | G | G | G | G | G | putative transcriptional activator ToxR |
| A | A | T | A | A | A | A | A | ||
| VCA1063 | G | G | G | G | T | T | G | G | Ornithine decarboxylase |
* = Not found in V. cholerae N16961.
Genomic Islands, Pathogenicity Islands, and Virulence Factors
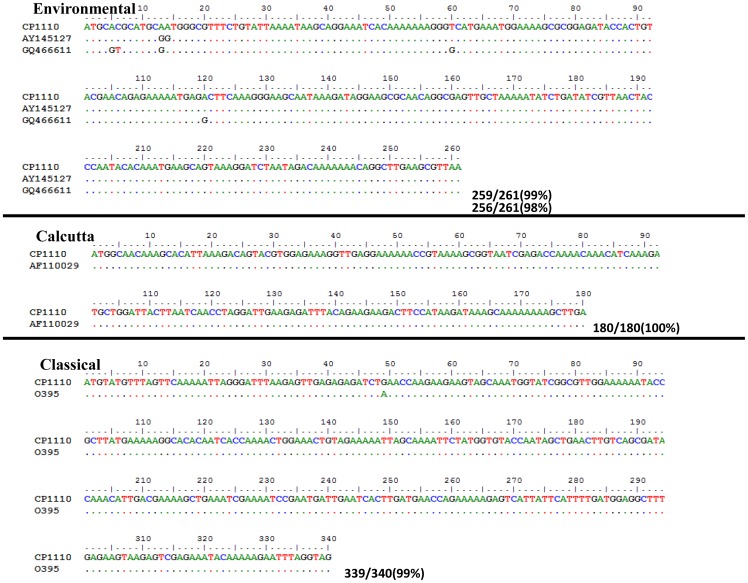
The V. cholerae FL Group isolates were determined to contain the full CTX phage encoding the cholera toxin, but the structure of this region was unresolved due to the limitations of assembly since ORFs were found on multiple contigs. For similar reasons, CTX phage copy number could not be resolved. A BLASTN analysis with V. cholerae N16961 and O395 as reference demonstrated the presence of regions homologous to VC1456 to VC1463 (VC0395_0512 to VC0395_0505 and VC0395_A1060 and VC0395_A1067 of V. cholerae O395) of the CTX phage (ctxB, ctxA, zot, ace, orfU,cep, rstB, rstA, and rstR Classical). To infer the biotype of the cholera toxin, PCR targeting the ctxB gene was employed and resulted in an amplicon for primers of targeting ctxB Classical. These PCR results are consistent with profiles of other clinically isolated V. cholerae strains on a global scale that suggest this cholera toxin biotype is the predominant biotype currently causing the majority of disease [34], [35]. Based on the genome sequence data, the CTX phage of the V. cholerae FL Group genomes were lacking the rstR gene of V. cholerae N16961 El Tor (VC1464), but did encode the rstR gene homologous to the one encoded in V. cholerae O395 Classical. To further investigate and confirm these in silico results, PCR targeting the rstR region was done and resulted in amplicons for the Calcutta, Environmental, and Classical biotypes, but not the El Tor biotype, an as-to-date uncommon combination. The rstR amplicons of CP1110 were subjected to Sanger sequencing and the resulting sequences were compared by BLASTN to the NCBI Genbank database for better interpretation of these results and each showed ≥99% nucleotide sequence similarity to Calcutta, Environmental, and Classical sequences (Figure 2). These amplicon sequences were compared with V. cholerae CP1110 reads by BLASTN to re-confirm their presence in the genome sequences. The rstR sequences from the V. cholerae FL Group were confirmed as Calcutta, Environmental, and Classical biotypes (Figure 2). The prototypical V. cholerae O1 El Tor strains encode rstR El Tor and ctxB El Tor while Classical strains encode rstR Classical and ctxB Classical. Altered V. cholerae O1 El Tor strains which differ from prototypical El Tor strains in their rstR/ctxB types have recently been identified [24]. Data from this study further demonstrates the diversity of the CTX phage outside of the more frequently studied V. cholerae O1 strains and suggests many alleles of this phage can be associated with cholera. Cholera toxin expression was not assayed in this study.
Figure 2. rstR sequences from V. cholerae CP1110.
Sequences are aligned to their most similar homologs extracted from NCBI Genbank database. Nulceotide sequence identity is shown to the right of the last nucleotide aligned for each allele.
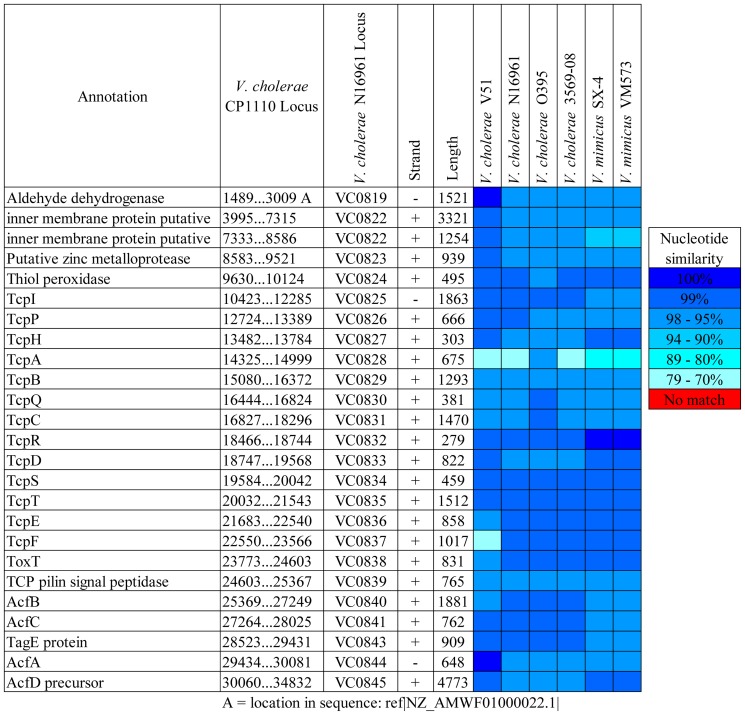
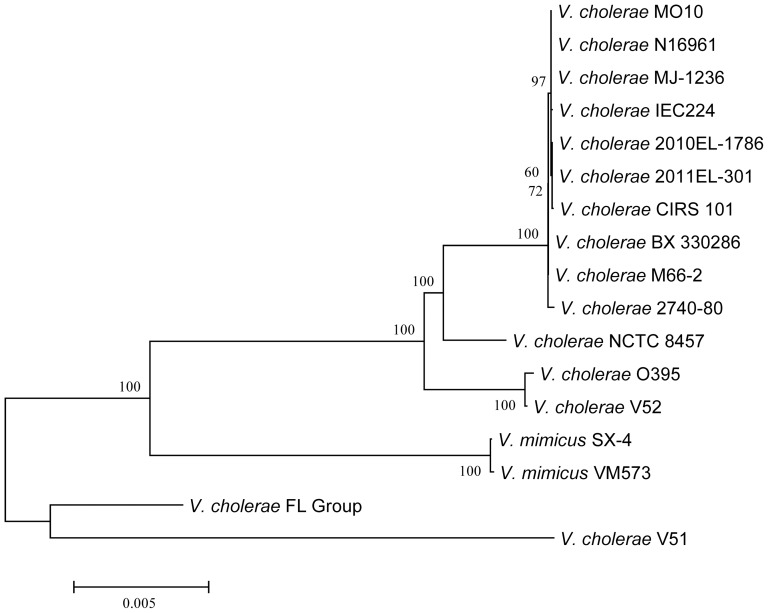
The genomes of the eight V. cholerae FL Group isolates harbored Vibrio pathogenicity island 1 (VPI-1) encoding the toxin co-regulated pilus (TCP) shown to be responsible for biofilm formation in the intestine and a receptor for CTXΦ phage [36], [37]. VPI-1 of the V. cholerae FL Group is highly similar in structure to those of other clinical and environmental V. cholerae and V. mimicus (Figure 3). Interestingly, the tcpA gene (often used as a marker of V. cholerae biotype) of this group has the highest similarity with that of V. cholerae O395, a Classical biotype, while showing similarity of 77% with V. cholerae V51. However, a phylogeny of concatenated ORFs of this island demonstrates VPI-1 of the V. cholerae FL Group and V. cholerae V51 are closely related to each other from an evolutionary perspective, and significantly diverged from VPI-1 of other clinical and environmental V. cholerae and V. mimicus strains (Figure 4).
Figure 3. Comparative genomic analysis of Vibrio pathogenicity island 1 (VPI-1).
VPI-1 of the V. cholerae FL Group is the reference sequence in a BLAST alignment with homologs of other Vibrionaceae genomes. Colored squares show degree of similarity.
Figure 4. Phylogenetic analysis of Vibrio pathogenicity island 1 (VPI-1).
Neighbor-joining tree showing evolutionary relationships of VPI-1. The calculation was based on aligned fragments of 25 orthologous genes (VC0819 to VC0845) comprising ca. 26.9 kb. Bar length = 0.005 substitutions per site.
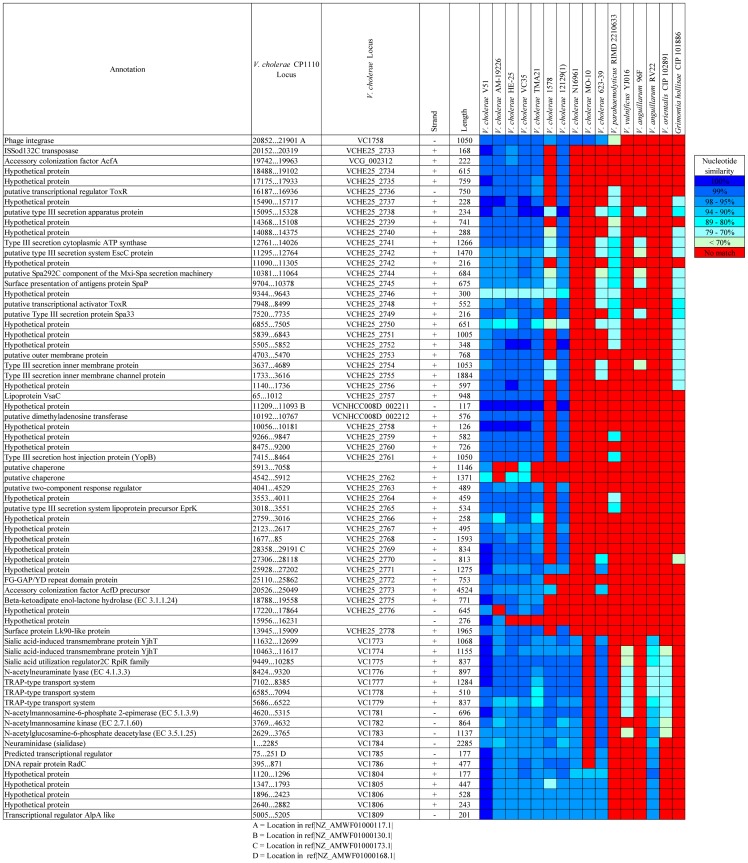
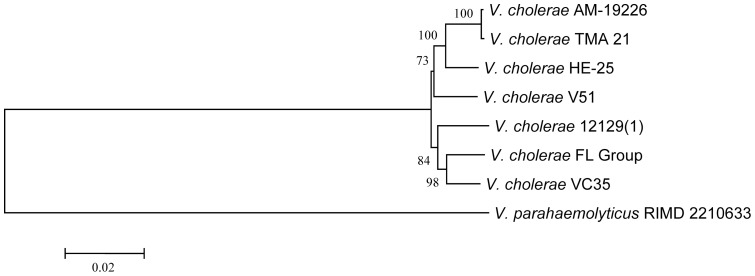
The genomes of all V. cholerae FL Group isolates also encoded VPI-2, with a type III secretion system (T3SS) (Figure 5). Two divergent T3SS variants have been identified in V. cholerae isolates [38]. T3SS in the V. cholerae FL Group genomes are most similar to that of V. cholerae V51 and AM-19226, a non-O1 TCP-negative and CTX-negative isolate (Figure 5). The T3SS of V. cholerae AM-19226 has been shown to be essential for colonization of the infant rabbit intestine and associated with severe diarrhea in this model, suggesting it plays a significant role in virulence during human infections [39]. This region has been found in environmental and clinical V. cholerae on a global scale. For instance, V. cholerae HE-25, a gray water isolate from Haiti and V. cholerae VC35, a clinical isolate from Malaysia, both encode T3SS that is structurally and phylogenetically similar to the variant in the V. cholerae FL Group suggesting global distribution of this virulence factor in environmental and clinical isolates (Figure 6). A phylogeny based on conserved ORFs of this variant and of V. parahaemolyticus as an outgroup infers the nearest phylogenetic neighbor to T3SS in the V. cholerae FL Group is V. cholerae VC35 (Figure 7). Although this region has been shown to be part of VPI-2 variants it has been identified as a separate genomic island capable of lateral transfer between V. cholerae strains [12], [40].
Figure 5. Comparative genomic analysis of Vibrio pathogenicity island 2 (VPI-2).
VPI-2 of the V. cholerae FL Group is the reference sequence in a BLAST alignment with homologs of other Vibrionaceae genomes. Colored squares show degree of similarity.
Figure 6. Phylogenetic analysis of ORFs conserved among all T3SS-positive V. cholerae and closely related species.
Neighbor-joining tree inferred from an alignment of 7 orthologous genes (VCHE25_2738, VCHE25_2741, VCHE25_2742, VCHE25_2744, VCHE25_2745, VCHE25_2749, VCHE25_2754) comprising ca. 5.2 kb. Bar length = 0.05 substitutions per site.
Figure 7. Phylogenetic analysis of most closely related T3SS.
Neighbor-joining tree inferred from an alignment of 17 orthologous genes (VCHE25_2737 to VCHE25_2742, VCHE25_2745 to VCHE25_2752, and VCHE25_2754) comprising ca. 10.2 kb. Bar length = 0.02 substitutions per site.
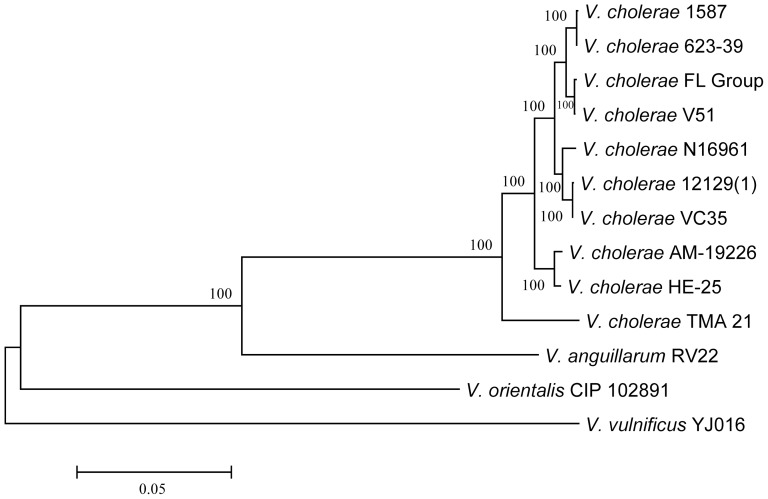
VPI-2 of the V. cholerae FL Group also encodes a complete sialic acid catabolism operon (homologs of VC1773 to VC1784 in the canonical VPI-2 of V. cholerae N16961), including a neuraminidase (sialidase) which has been shown to unmask the GM1 gangliosides of human intestinal epithelial cells, making them available to the cholera toxin [10]. A phylogeny of the sialic acid metabolism region demonstrated this operon in the V. cholerae FL Group is closely related to that of V. cholerae V51, V. cholerae 1587, and V. cholerae 623-39 (Figure 8). The phylogeny of this region is not congruent with that of the T3SS suggesting a more recent ancestral sialic acid metabolism region of the V. cholerae FL Group and V. cholerae V51 than that of the T3SS. Further, when the phylogenies of T3SS and sialic acid metabolism operons of seven V. cholerae strains with homologous ORFs are inferred (V. cholerae strains AM-19226, TMA 21, HE-25, V51, 12129(1), FL Group, and VC35), sister taxa of the V. cholerae FL Group T3SS remains V. cholerae VC35 and sister taxa of the sialic acid metabolism region of the V. cholerae FL Group remains V. cholerae V51 (data not shown). These data suggest the two regions in the V. cholerae FL Group originated from different sources. Morita et al. [40] demonstrated these two regions of VPI-2 could be of separate origin and the insertion locus of V. cholerae T3SS is exclusively in VPI-2. Interestingly, the mu-like phage region, the most variable region of the canonical VPI-2, is absent in these genomes.
Figure 8. Phylogenetic analysis of sialic acid metabolism region of VPI-2.
Neighbor-joining tree inferred from an alignment of 7 orthologous genes (VC1781 to VC1774) comprising ca. 5.7 kb. Bar length = 0.05 substitutions per site.
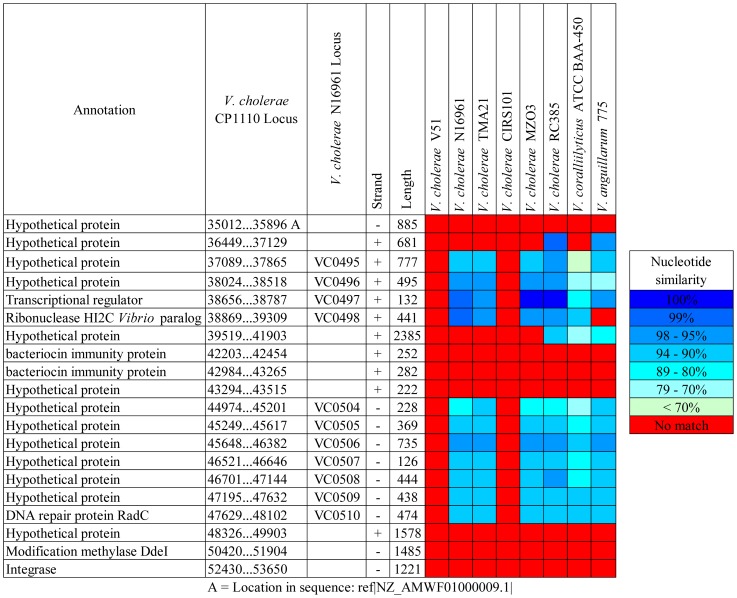
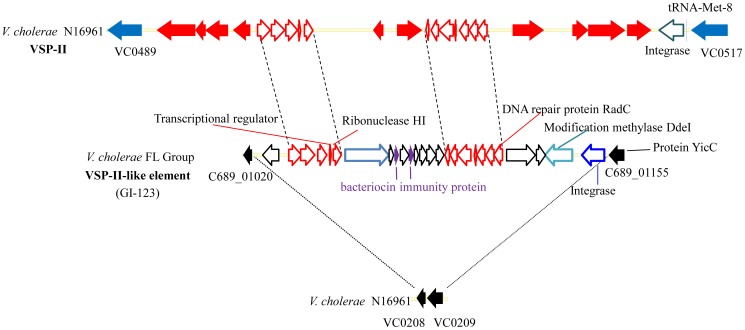
A VSP-II-like island was identified in the V. cholerae FL Group isolates with varying levels of similarity and conservation with other homologous sequences in the Vibrionaceae (Figure 9). This island was previously identified as GI-123, but was not well characterized [41]. Interestingly, this island does not encode the canonical integrase of VSP-II but rather one that is similar to an integrase of a not yet described genomic island in V. cholerae CP1033(6), a serogroup O1 strain isolated from a cholera patient in Mexico in 2000. This VSP-II-like island was not inserted at the tRNA-Met (adjacent to VC0517) where the canonical VSP-II is inserted, but rather at the locus homologous to VC0208 and VC0209, where GIs-32, 52, 68, 96, 98, 107 are inserted in other V. cholerae strains [12], [41]. When compared to the prototypical VSP-II island in V. cholerae N16961, the V. cholerae FL Group encodes two regions with high similarity: VC0495 to VC0498 and VC0504 to VC0510. A novel region encoding four ORFs annotated as hypothetical protein, bacteriocin immunity protein, bacteriocin immunity protein, and hypothetical protein were inserted between the two regions that are similar to the prototypical VSP-II (Figures 9 and 10). One of these hypothetical proteins comprises 794 amino acids, with cytoxic and S-type Pyocin domains, known toxins active against bacteria [42]. When compared to the NCBI nucleotide database, highest similarity is with an S-type Pyocin domain-containing protein (YP_004564713.1) of V. anguillarum, a marine fish pathogen. Two adjacent proteins are bacteriocin immunity proteins, with one 83 amino acids and the other 93 amino acids in length. Both have colicin immunity protein/pyocin immunity protein domains and are predicted by pSort to be in the cytoplasm of the V. cholerae [43]. In other species secreted pyocins are known to cause cell death among closely related strains [42]. The presence of a homologous genetic cluster in the V. cholerae FL Group may allow it to outcompete other V. cholerae strains present in the same local environment which may lead to an increased density of pyocin and pyocin immunity protein-encoding strains in a specific environment such as a single oyster bed. However, further research on pyocins in V. cholerae needs to be conducted to further elucidate their potential role in intra-species competition in the environment.
Figure 9. Comparative genomic analysis of Vibrio Seventh pandemic island II-like island.
Vibrio Seventh pandemic II-like island of the V. cholerae FL Group is the reference sequence in a BLAST alignment with homologs of other Vibrionaceae genomes. Colored squares show degree of similarity.
Figure 10. Structure of the canonical Vibrio Seventh Pandemic island II (VSP-II) and the VSP-II-like element of the V. cholerae FL Group.
Structure of the canonical VSP-II element of V. cholerae N16961 (top), the VSP-II-like element found in the V. cholerae FL Group (middle) based on the annotation in NCBI Genbank, and the homologous locus of insertion of this VSP-II-like element in V. cholerae N16961 (bottom). Sequences homologous to VC0208 and VC0209 in V. cholerae V51 are found on contig NZ_DS179740 at positions 34343 to 34831 and 34897 to 35763. ORFs conserved between the two elements are outlined in red.
The VSP-II-like element in isolates of the V. cholerae FL Group has 12 ORFs with similarity to regions of the V. corallilyticus ATCC BAA-450 and V. anguillarum 775 genomes, with percent nucleotide identity between the ORFs ranging from 69 to 99% (Figure 9). These data suggest the suite of VSP-II-like elements is distributed not only among clinical V. cholerae isolates, but also environmental isolates including non-cholera vibrios. Further, the presence of similar ORFs in non-pathogenic vibrios strongly indicates a function in the natural environment.
A phylogeny of conserved VSP-II ORFs infers these sequences of the V. cholerae FL Group to be closely related to V. cholerae TMA 21 and significantly divergent from the V. cholerae 7th Pandemic strains (Figure 11). The subclade with which VSP-II of the V. cholerae FL Group clusters comprises environmental V. cholerae strains and Vibrio sp. RC341, a novel Vibrio species closely related to V. cholerae and known to cause sporadic infections in humans [15], [44], [45]. V. cholerae V51 does not encode this element.
Figure 11. Phylogenetic analysis of Vibrio Seventh pandemic island II-like island.
Neighbor-joining tree inferred from an alignment 8 orthologous ORFs (VC0495, VC0496, VC0504 to VC0506, VC0508 to VC0510) comprising ca. 3.7 kb. Bar represents 0.02 substitutions per site.
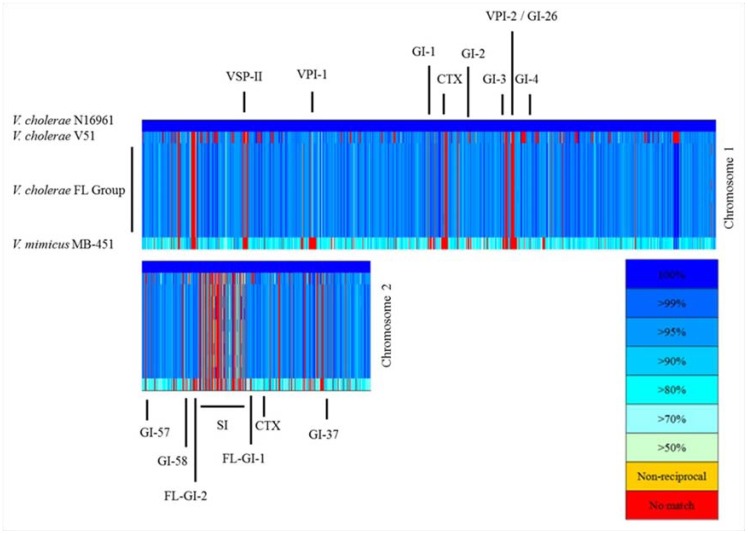
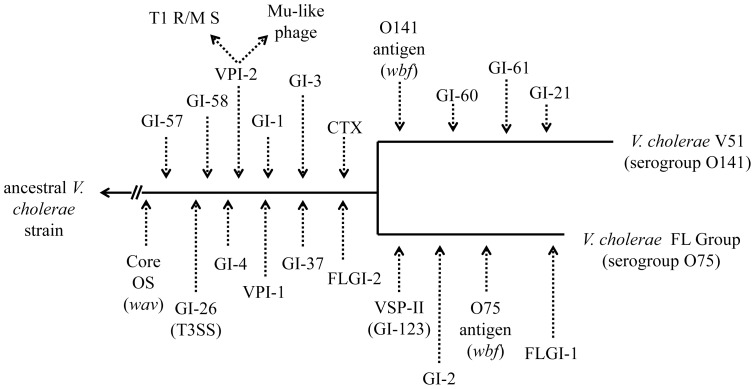
The presence of genomic islands comprising the V. cholerae mobilome described by Chun et al. (12) was evaluated using BLASTN and BLASTP. Including VPI-1 and 2 and a VSP-II-like element, the V. cholerae FL Group encoded sequences with high similarity to GIs-1, 2, 3, 4, 26, 37, 57, 58, and two genomic islands not yet described and designated here as FL-GI-1 and FL-GI-2 (Figure 12). All V. cholerae FL Group isolates lacked VSP-I genomic island and the site of insertion does not harbor any other genomic island. Figure 13 depicts a proposed arrangement of genomic island insertion and deletion in the V. cholerae V51/V. cholerae FL Group lineage before and after the two sets of isolates (V. cholerae V51 and V. cholerae FL Group isolates) would have diverged from a common ancestor.
Figure 12. BLAST atlas of V. cholerae FL Group, V. cholerae V51, and V. mimicus MB-451 and V. cholerae N16961 genomes.
V. cholerae N16961 is the reference genome. Genomic islands with the prefix “GI” are described by Chun et al. [12]. SI = superintegron.
Figure 13. Proposed hypothetical insertions of genomic islands in the V. cholerae V51/V. cholerae FL Group clade.
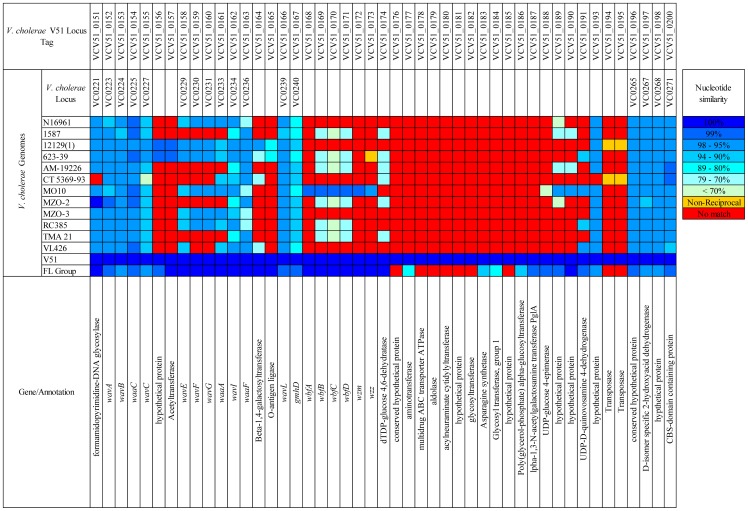
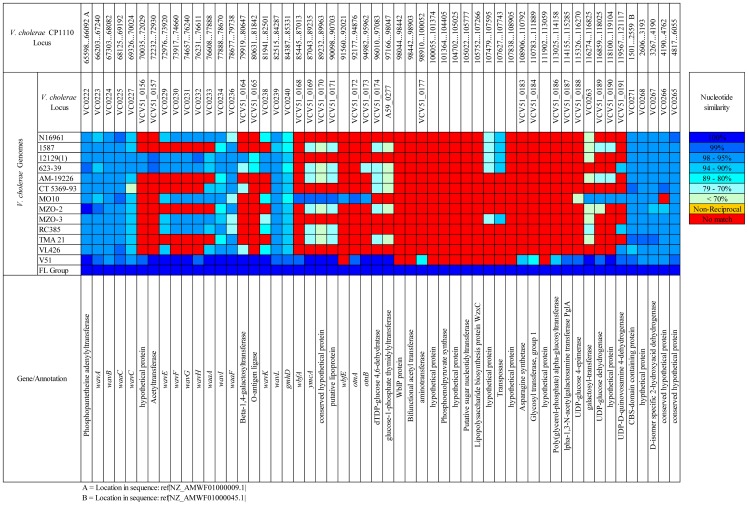
Lipopolysaccharide Coding Region
This region of the V. cholerae FL Group is ca. 60.1 kb, with the LPS core region ca. 19.1 kb and wb* region ca. 41 kb. Of all V. cholerae serogroup data represented in NCBI GenBank, the core oligosaccharide (OS) and the O141-antigen-specific coding regions of V. cholerae V51 are most similar to the homologous ORFs of V. cholerae FL Group (Figures 14 and 15). Of 54 identified ORFs in this region of the V. cholerae FL Group, V. cholerae V51 shares 38 with at least 95% nucleotide sequence similarity. When the O-antigen ORFs of V. cholerae V51 and the V. cholerae FL Group are compared the only observed structural differences are seven ORFs absent in the regions homologous to VCV51_0176 to VCV51_0185 in the V. cholerae FL Group and 11 ORFs in V. cholerae V51 absent in the homologous region (found between positions 98044 and 113059 in contig ref|NZ_AMWF01000009.1|). Eight ORFs were identified in the O75-antigen coding region of the V. cholerae FL Group isolates that have not yet been described in the O-antigen coding regions of other V. cholerae genomes, and these ORFs may be specific to the O75 antigen (Figures 14 and 15).
Figure 14. Comparative genomic analysis of LPS coding regions.
Reciprocal BLAST analysis of LPS coding region with V. cholerae V51 as a reference.
Figure 15. Comparative genomic analysis of LPS coding regions.
Reciprocal BLAST analysis of LPS coding region with V. cholerae FL Group as a reference.
Although it is well known that this region is a hot-spot for gene transfer, it can be assumed that O141 and O75 O-antigen coding regions derived from a recent ancestral sequence based on the high level of conservation between the two, and that the difference between the two clusters arises from a substitution of ORFs specific to the O-antigen region. A similar mechanism has been suggested for the relationship between O139 and O22 serogroups [46], [47]. This substitution may have involved a ca. 18.2 kb region in the genomes of V. cholerae FL Group isolates and a ca. 16.2 kb region in V. cholerae V51 flanked by homologs found at nucleotide positions 97166 to 98047 (glucose-1-phosphate thymidylyltransferase) and 116274 to 116825 (lipid carrier:UDP-N-acetylgalactosaminyltransferase). Alternatively, three substitution events involving shorter sequences may have occurred between the flanking regions, indicated by absent ORFs (red squares in Figure 15) in reciprocal comparison. Interestingly, the serogroup with the next highest level of conservation with serogroups O141 and O75 is the epidemic-associated O139 serogroup isolate V. cholerae MO10.
Phenotypic Analyses
The eight V. cholerae FL Group isolates were evaluated for hemolysis, motility, and proteolysis, following standard methods for testing these methods in V. cholerae [27]. Although not responsible for the rice water diarrhea characteristic of cholera, these virulence factors are associated with intestinal and extra-intestinal V. cholerae infections, as well as ecological functions in the aquatic environment [48], [49], [50], [51]. All strains are motile, proteolytic, form biofilms and are hemolytic. However, strain CP1114 demonstrated weak or incomplete hemolysis. This isolate was also weakly proteolytic, compared to the other V. cholerae FL Group isolates, and incomplete hemolysis may be due to incomplete processing of hemolysin by the hemagglutinin/protease [52].
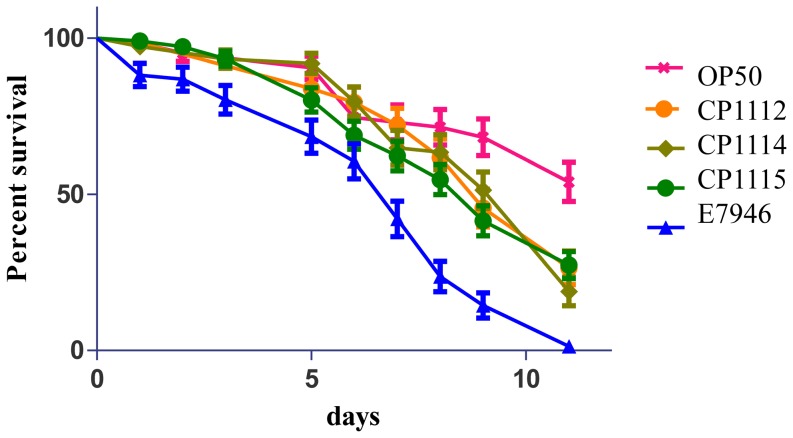
The Caenorhabditis elegans model of V. cholerae infection, which yields data on strength of hemolytic activity (hlyA) proved useful [29]. Nematodes were fed three isolates of V. cholerae FL Group (V. cholerae CP1112, 1114, and 1115). CP1115, which showed the largest zone of hemolysis on blood agar, was selected for testing. CP1114 demonstrated incomplete hemolysis and CP1112 showed a moderate zone of clearing when compared to the other isolates of the V. cholerae FL Group. The results demonstrated significantly more rapid lethality in nematodes fed the V. cholerae FL Group isolates than nematodes fed non-pathogenic E. coli as a control, but significantly slower lethality than nematodes fed V. cholerae El Tor strain E7946 (P<0.05) (Figure 16). It is concluded that all three of the V. cholerae FL Group isolates produced in similar C. elegans survival patterns. However, median survival time of worms fed isolates V. cholerae CP1112 and CP1115 was ca. nine days versus ca. eleven days for worms fed V. cholerae CP1114, the isolate with incomplete hemolysis, a consistent result based on previous observations. Interestingly, the three isolates caused a C. elegans die-off similar to V. cholerae O1 biotype Classical than to El Tor [29], not expected since hlyA of the V. cholerae FL Group does not have the same 11 bp deletion linked to the decreased hemolytic activity of V. cholerae O1 Classical but higher nucleotide sequence similarity with V. cholerae O1 El Tor N16961 than Classical O395.
Figure 16. Survival curves of C. elegans challenged with V. cholerae CP1112, CP1114, CP1115, V. cholerae El Tor E7946, Escherichia coli OP50.
Based on BiOLOG phenotypic microarray assay, all strains utilized sialic acid three to six times greater than background demonstrating a functional sialic acid catabolism operon of the VPI-2. Almagro-Moreno and Boyd [10] reported the ability to utilize sialic acid confers a competitive advantage to strains encoding this region during infection of the sialic acid-rich environment of the gut. This is due to the ability of V. cholerae encoding a functional sialic acid metabolism region to utilize sialic acid as a carbon source [10]. All strains also utilized maltose, which was shown by Lång et al. [53] to be related to cholera toxin and toxin co-regulated pilus production and translocation across the V. cholerae outer membrane. Results of the study demonstrated that a functional maltose operon is needed for virulence of V. cholerae [53].
The BiOLOG profiles showed similar metabolic profiles among the V. cholerae FL Group strains (data not shown). However, both replicates of V. cholerae CP1110 utilized caproic acid as carbon source while all other isolates generally did not, except isolate V. cholerae CP1117 which utilized this substrate in one replicate. Isolates CP1112, CP1113, and CP1116 weakly utilized caproic acid in at least one replicate. Isolate V. cholerae CP1115 did not utilize β-methyl-D-glucoside while the other V. cholerae FL Group isolates did.
Conclusions
It is concluded the outbreak was caused by V. cholerae growing to a sufficiently high density in the environment (i.e., not in a single oyster) to cause multiple cases of cholera. Clonality of the isolates, including 67% of all reported cholera cases from this outbreak, demonstrates that there need not be a human vehicle of V. cholerae dispersal into a given geographical region prior to a cholera outbreak, as has been suggested for cholera epidemics. Further, it is concluded that genomic and phenotypic diversity exists among clinical isolates V. cholerae non-O1/non-O139 strains of the same outbreak, supporting a recommendation to investigate the genomics of cholera epidemics at the population level. Large-scale genomic and molecular analyses accomplished for the cholera epidemics in Haiti and Bangladesh and the recent epidemics in Nigeria and Kenya have revealed distinct V. cholerae populations causing disease [6], [7], [54], [55].
Because the V. cholerae FL Group isolates formed a monophyletic lineage with V. cholerae V51 serogroup O141 (a 1987 clinical isolate), we hypothesize the clade to represent a lineage of cholera-causing isolates, similar to those of the 7th pandemic clade. Although, diverged from recent 7th pandemic strains and older Classical and pre-7th pandemic strains, from an evolutionary perspective, the virulence factors known to be involved in cholera are present in the V. cholerae FL Group and V. cholerae V51. The difference in the constellation of mobile elements and incongruent phylogenies of some elements of V. cholerae V51 and the V. cholerae FL Group suggest that, although these two groups are similar, they have independently acquired various elements from the environment, with some islands globally distributed.
Although the majority of the research on V. cholerae focuses on the O1 serogroup because of the major epidemics associated with these strains, V. cholerae non-O1/non-O139 serogroup strains should be further evaluated for contribution to the global disease burden. V. cholerae serogroup O141 isolates have been shown by other investigators to globally cause significant disease and many encode ctxB Classical [14], [30], [31], [56] as do the V. cholerae FL Group serogroup O75 isolates. Pathogenic V. cholerae causing cholera outbreaks must be characterized in a phylogenomic context and their genomic island constellations as well. It is no longer sufficient to label these V. cholerae strains as serogroups O1, O139, or non-O1/non-O139, without further appropriate genomic analysis.
Funding Statement
This work was supported by a grant the National Science Foundation (www.nsf.gov), No. 0813066, National Institutes of Health (www.nih.gov) Grant 2RO1A1039129-11A2, National Institutes of Health-Fogarty International Center (http://www.fic.nih.gov) Challenge Grant 1RC1TW008587-01 and National Oceanic and Atmospheric Administration (www.noaa.gov) Grant No. SO660009. The funders provided salary and laboratory supplies only. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Ko WC, Chuang YC, Huang GC, Hsu SY (1998) Infections due to non-O1 Vibrio cholerae in southern Taiwan: predominance in cirrhotic patients. Clin Infect Dis 27: 774–780. [DOI] [PubMed] [Google Scholar]
- 2. Safrin S, Morris JG Jr, Adams M, Pons V, Jacobs R, et al. (1988) Non-O:1 Vibrio cholerae bacteremia: case report and review. Rev Infect Dis 10: 1012–1017. [DOI] [PubMed] [Google Scholar]
- 3. Shannon JD, Kimbrough RC (2006) Pulmonary Cholera Due to Infection with a Non-O1 Vibrio cholerae Strain. J Clin Microbiol 44: 3459–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lukinmaa S, Mattila K, Lehtinenc V, Hakkinen M, Koskela M, et al. (2006) Territorial waters of the Baltic Sea as a source of infections caused by Vibrio cholerae non-O1, non-O139: report of 3 hospitalized cases. Diagn Micr Infec Dis 54: 1–6. [DOI] [PubMed] [Google Scholar]
- 5. Chatterjee S, Ghosh K, Raychoudhuri A, Chowdhury G, Bhattacharya MK, et al. (2009) Incidence, virulence factors, and clonality among clinical strains of non-O1, non-O139 Vibrio cholerae isolates from hospitalized diarrheal patients in Kolkata, India. J Clin Microbiol 47: 1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hasan NA, Choi SY, Eppinger M, Clark PW, Chen A, et al. (2012) Genomic diversity of 2010 Haitian cholera outbreak strains. Proc Natl Acad Sci USA 109(29): E2010–E2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marin MA, Thompson CC, Freitas FS, Fonseca EL, Aboderin AO, et al. (2013) Cholera Outbreaks in Nigeria Are Associated with Multidrug Resistant Atypical El Tor and Non-O1/Non-O139 Vibrio cholerae . PLoS Negl Trop Dis 7 ((2)) e2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tacket C, Taylor R, Losonsky G, Lim Y, Nataro J, et al. (1998) Investigation of the Roles of Toxin-Coregulated Pili and Mannose-Sensitive Hemagglutinin Pili in the Pathogenesis of Vibrio cholerae O139 Infection. Infect Immun 66 ((2)) 692–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vanden Broeck D, Horvath C, De Wolf M (2007) Vibrio cholerae: Cholera toxin. Int J Biochem Cell Biol 39 ((10)) 1771–1775. [DOI] [PubMed] [Google Scholar]
- 10. Almagro-Moreno S, Boyd EF (2009) Sialic Acid Catabolism Confers a Competitive Advantage to Pathogenic Vibrio cholerae in the Mouse Intestine. Infect Immun 77 ((9)) 3807–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manning S, Motiwala A, Springman A, Qi W, Lacher D, et al. (2008) Variation in virulence among clades of Escherichia coli O157:H7 associated with disease outbreaks. Proc Natl Acad Sci USA ((12)) 4868–4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chun J, Grim CJ, Hasan NA, Lee JH, Choi SY, et al. (2009) Comparative Genomics Reveals Mechanism for Short-term and Long-term Clonal Transitions in Pandemic Vibrio cholerae . Proc Natl Acad Sci USA 106: 15442–15447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meibom K, Li X, Nielsen A, Wu C, Roseman S, et al. (2004) The Vibrio cholerae chitin utilization program. Proc Natl Acad Sci USA 101 ((8)) 2524–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Udden S, Zahid M, Biswas K, Ahmad Q, Cravioto A, et al. (2008) Acquisition of classical CTX prophage from Vibrio cholerae O141 by El Tor strains aided by lytic phages and chitin-induced competence. Proc Natl Acad Sci USA 105 ((33)) 11951–11956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boucher Y, Cordero OX, Takemura A, Hunt DE, Schliep K, et al. (2011) Local mobile gene pools rapidly cross species boundaries to create endemicity within global Vibrio cholerae populations. mBio 2 ((2)) e00335–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hlady W, Klontz K (1996) The Epidemiology of Vibrio Infections in Florida, 1981–1993. J Infect Dis 173 ((5)) 1176–1183. [DOI] [PubMed] [Google Scholar]
- 17. Shapiro R, Altekruse S, Hutwagner L, Bishop R, Hammond R, et al. (1998) The Role of Gulf Coast Oysters Harvested in Warmer Months in Vibrio vulnificus Infections in the United States, 1988–1996. J Infect Dis 178 ((3)) 752–759. [DOI] [PubMed] [Google Scholar]
- 18. Tamplin M (2001) Coastal Vibrios: Identifying Relationships between Environmental Condition and Human Disease. Human and Ecological Risk Assessment: An International Journal 7 ((5)) 1437–1445. [Google Scholar]
- 19. Lipp E, Huq A, Colwell R (2002) Effects of Global Climate on Infectious Disease: the Cholera Model. Clin Microbiol Rev 15 ((4)) 757–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huq A, Sack R, Nizam S, Longini I, Nair G, et al. (2005) Critical Factors Influencing the Occurrence of Vibrio cholerae in the Environment of Bangladesh. Appl Environ Microbiol 71 ((8)) 4645–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tobin-D'Angelo M, Smith A, Bulens S, Thomas S, Hodel M, et al. (2008) Severe Diarrhea Caused by Cholera Toxin–Producing Vibrio cholerae Serogroup O75 Infections Acquired in the Southeastern United States. Clin Infect Dis 47 ((8)) 1035–1040. [DOI] [PubMed] [Google Scholar]
- 22. Onifade T, Hutchinson R, Van Zile K, Bodager D, Baker R, et al. (2011) Toxin producing Vibrio cholerae O75 outbreak, United States, March to April 2011. Euro Surveill 16 ((20)) 19870. [PubMed] [Google Scholar]
- 23. Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, et al. (2008) The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi SY, Lee JH, Kim EJ, Lee HR, Jeon Y, et al. (2010) Classical RS1 and environmental RS1 elements in Vibrio cholerae O1 El Tor strains harbouring a tandem repeat of CTX prophage: revisiting Mozambique in 2005. J Med Microbiol 59: 302–308. [DOI] [PubMed] [Google Scholar]
- 25. Vora G, Meador M, Bird M, Bopp C, Andreadis J, et al. (2005) Microarray-based detection of genetic heterogeneity, antimicrobial resistance, and the viable but nonculturable state in human pathogenic Vibrio spp. Proc Natl Acad Sci USA 102 ((52)) 19109–19114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nusrin S, Khan GY, Bhuiyan NA, Ansaruzzaman M, Hossain MA, et al. (2004) Diverse CTX phages among toxigenic Vibrio cholerae O1 and O139 strains isolated between 1994 and 2002 in an area where cholera is endemic in Bangladesh. J Clin Microbiol 42: 5854–5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Son MS, Taylor RK (2011) Genetic Screens and Biochemical Assays to Characterize Vibrio cholerae O1 Biotypes: Classical and El Tor. Curr Protoc Microbiol 22: 6A.2.1–6A.2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sprando R, Olejnik N, Cinar H, Ferguson M (2009) A method to rank order water soluble compounds according to their toxicity using Caenorhabditis elegans, a Complex Object Parametric Analyzer and Sorter, and axenic liquid media. Food and Chemical Toxicology 47 ((4)) 722–728. [DOI] [PubMed] [Google Scholar]
- 29. Cinar HN, Kothary M, Datta AR, Tall BD, Sprando R, et al. (2010) Vibrio cholerae Hemolysin Is Required for Lethality, Developmental Delay, and Intestinal Vacuolation in Caenorhabditis elegans . PLoS ONE 5 ((7)) e11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dalsgaard A, Serichantalergs O, Forslund A, Lin W, Mekalanos J, et al. (2001) Clinical and Environmental Isolates of Vibrio cholerae Serogroup O141 Carry the CTX Phage and the Genes Encoding the Toxin-Coregulated Pili. J Clin Microbiol 39 ((11)) 4086–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Crump JA, Bopp CA, Greene KD, Kubota KA, Middendorf RL, et al. (2003) Toxigenic Vibrio cholerae serogroup O141-associated cholera-like diarrhea and bloodstream infection in the United States. J Infect Dis 187 ((5)) 866–8. [DOI] [PubMed] [Google Scholar]
- 32. Blake PA, Allegra DT, Snyder JD, Barrett TJ, McFarland L, et al. (1980) Cholera–a possible endemic focus in the United States. N Engl J Med 302 ((6)) 305–309. [DOI] [PubMed] [Google Scholar]
- 33. Lin FY, Morris JG Jr, Kaper JB, Gross T, Michalski J, et al. (1986) Persistence of cholera in the United States: isolation of Vibrio cholerae O1 from a patient with diarrhea in Maryland. J Clin Microbiol 23 ((3)) 624–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nair GB, Qadri F, Holmgren J, Svennerholm AM, Safa A, et al. (2006) Cholera due to altered El Tor strains of Vibrio cholerae O1 in Bangladesh. J Clin Microbiol 44: 4211–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Safa A, Sultana J, Cam PD, Mwansa JC, Kong RYC (2008) Classical cholera toxin producing Vibrio cholerae O1 hybrid El Tor strains in Asia and Africa. Emerg Infect Dis 14: 987–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Waldor MK, Mekalanos JJ (1996) Lysogenic conversion by a filamentous phage encoding cholera toxin. Science 272 ((5270)) 1910–4. [DOI] [PubMed] [Google Scholar]
- 37. Reguera G, Kolter R (2005) Virulence and the environment: a novel role for Vibrio cholerae toxin-coregulated pili in biofilm formation on chitin. J Bacteriol 187 ((10)) 3551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Murphy RA, Boyd EF (2008) Three pathogenicity islands of Vibrio cholerae can excise from the chromosome and form circular intermediates. J Bacteriol 190 ((2)) 636–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shin OS, Tam VC, Suzuki M, Ritchie JM, Bronson RT, et al. (2011) Type III secretion is essential for the rapidly fatal diarrheal disease caused by non-O1, non-O139 Vibrio cholerae . mBio 2 ((3)) e00106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morita M, Yamamoto S, Hiyoshi H, Kodama T, Okura M, et al. (2013) Horizontal gene transfer of a genetic island encoding a type III secretion system distributed in Vibrio cholerae . Microbiol Immunol 57 ((5)) 334–9. [DOI] [PubMed] [Google Scholar]
- 41.Choi SY, Hasan NA, Chun J, Hoq M, Huq A, et al.. (2011) Comparative Genomic MGE finding process streamlines mobile element search. ASM Biodefense and Emerging Pathogens Research Meeting. Washington, DC.
- 42. Michel-Briand Y, Baysse C (2002) The pyocins of Pseudomonas aeruginosa . Biochimie 84 ((5–6)) 499–510. [DOI] [PubMed] [Google Scholar]
- 43. Nakai K, Horton P (1999) PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci 24 ((1)) 34–35. [DOI] [PubMed] [Google Scholar]
- 44. Haley BJ, Grim CJ, Hasan NA, Choi SY, Chun J, et al. (2010) Comparative genomic analysis reveals evidence of two novel Vibrio species closely related to V. cholerae . BMC Microbiol 10: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Taviani E, Grim CJ, Choi J, Chun J, Haley B, et al. (2010) Discovery of novel Vibrio cholerae VSP-II genomic islands using comparative genomic analysis. FEMS Microbiol Lett 308 ((2)) 130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dumontier S, Berche P (1998) Vibrio cholerae O22 might be a putative source of exogenous DNA resulting in the emergence of the new strain of Vibrio cholerae O139. FEMS Microbiol Lett 164 ((1)) 91–98. [DOI] [PubMed] [Google Scholar]
- 47. Yamasaki S, Shimizu T, Hoshino K, Ho S, Shimada T, et al. (1999) The genes responsible for O-antigen synthesis of Vibrio cholerae O139 are closely related to those of Vibrio cholerae O22. Gene 237 ((2)) 321–332. [DOI] [PubMed] [Google Scholar]
- 48. Halpern M, Gancz H, Broza M, Kashi Y (2003) Vibrio cholerae hemagglutinin/protease degrades chironomid egg masses. Appl Environ Microbiol 69 ((7)) 4200–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Krukonis ES, DiRita VJ (2003) From motility to virulence: sensing and responding to environmental signals in Vibrio cholerae . Curr Opin Microbiol 6: 186–190. [DOI] [PubMed] [Google Scholar]
- 50. Silva AJ, Leitch GJ, Camilli A, Benitez JA (2006) Contribution of Hemagglutinin/Protease and Motility to the Pathogenesis of El Tor Biotype Cholera. Infect Immun 74 ((4)) 2072–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shinoda S (2011) Proteases Produced by Vibrio cholerae and Other Pathogenic Vibrios: Pathogenic Roles and Expression. In Epidemiological and Molecular Aspects on Cholera, Infectious Disease. Ramamurthy, T., Bhattacharya, S.K. (eds.) New York, NY: Springer Science, pp. 245–258. [Google Scholar]
- 52. Hall RH, Drasar BS (1990) Vibrio cholerae HlyA hemolysin is processed by proteolysis. Infect Immun 58 ((10)) 3375–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lång H, Jonson G, Holmgren J, Palva ET (1994) The maltose regulon of Vibrio cholerae affects production and secretion of virulence factors. Infect Immun 62 ((11)) 4781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ghosh R, Nair GB, Tang L, Morris JG, Sharma NC, et al. (2008) Epidemiological study of Vibrio cholerae using variable number of tandem repeats. FEMS Microbiol Lett 288: 196–201. [DOI] [PubMed] [Google Scholar]
- 55. Mohamed AA, Oundo J, Kariuki SM, Boga HI, Sharif SK, et al. (2012) Molecular epidemiology of geographically dispersed Vibrio cholerae O1, Kenya, January 2009–May 2010. Emerg Infect Dis 18 ((6)) 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Octavia S, Salim A, Kurniawan J, Lam C, Leung Q, et al. (2013) Population Structure and Evolution of Non-O1/Non-O139 Vibrio cholerae by Multilocus Sequence Typing. PLoS ONE 8 ((6)) e65342. [DOI] [PMC free article] [PubMed] [Google Scholar]