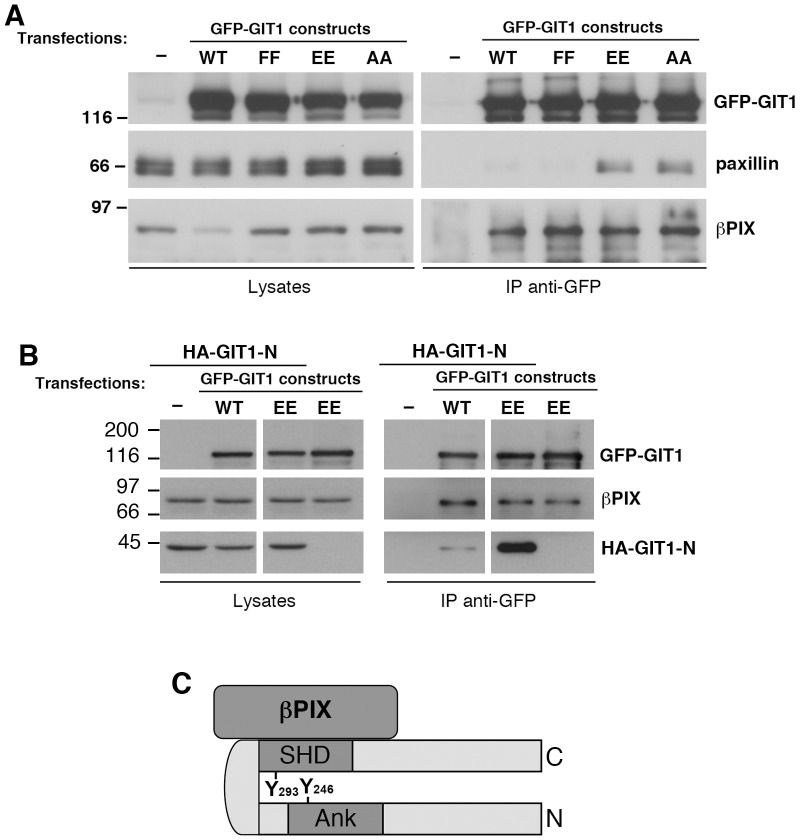
Figure 2. Mutation of tyrosines 246 and 293 does not affect the binding of GIT1 to βPIX.
(A) Lysates from mock-transfected cells or from cells transfected with the indicated GIT1 constructs were immunoprecipitated for GIT1 with anti-GFP antibody. Filters with immunoprecipitates (IP, from 200 μg of protein lysate), and lysates (25 μg) were blotted with anti-GFP (for GIT1), anti-paxillin, and anti-βPIX antibodies. (B) Binding of GIT1-N to GIT1-Y246E/Y293E does not affect the interaction of GIT1-Y246E/Y293E to βPIX. Lysates from COS7 cells co-transfected to express the HA-GIT1-N fragment and the full length GFP-GIT1-WT or GIT1-Y246E/Y293E proteins were immunoprecipitated (IP, from 175 μg of protein lysate) with anti-GFP. Lysates (25 μg, oƒn the lef) and IP (right) were blotted to reveal the full length proteins (anti-GFP), the HA-GIT1-N fragment (anti-HA mAb 12CA5), and endogenous βPIX. (C) Our data support the hypothesis that the tyrosine 293 of the SHD domain of GIT1 is required for the intramolecular interaction, but not for the interaction of the SHD domain of GIT1 with βPIX.