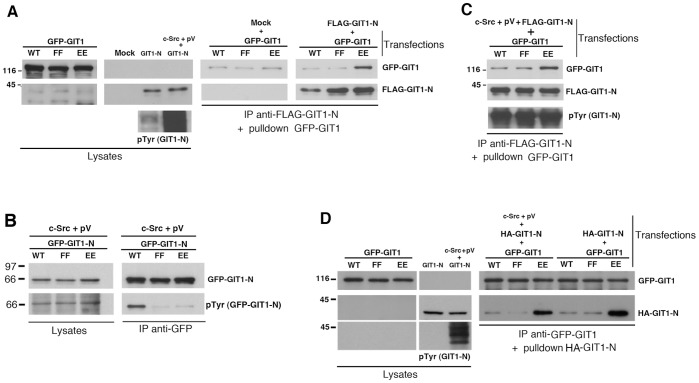
Figure 5. Hyperphosphorylation of GIT1-N by Src and pervanadate does not affect its binding in vitro to full length GIT1 proteins.
(A–C) COS7 cells were transfected with full length GFP-GIT1 or GIT1-N constructs (WT, FF, or EE), or with wildtype or mutant FLAG-GIT1-N fragments, alone or together with c-Src. The cells cotransfected with c-Src were incubated 20 min at 37°C with 1 mM pervanadate before lysis (pV). Aliquots of the lysates (200 μg of protein) were immunoprecipitated (IP) with anti-FLAG (A,C) or anti-GFP (B) antibodies. For pulldowns shown in (A) and (C): FLAG-immunoprecipitates were washed and incubated for 2 h at 4°C with lysates (250 μg of protein) from cells transfected with the indicated full length GFP-GIT1 constructs. Equal amounts of lysates (25 μg of protein), and the pulldowns performed with GIT1-N without (A) or with c-Src and pervanadate treatment (C) were blotted for the detection of the indicated antigens. In (B) the immunoprecipitations with anti-GFP antibody (right) were immunoblotted to detect the levels of GFP-GIT1-N protein (upper filter) and of its tyrosine phosphorylation (lower filter). (D) COS7 cells were transfected to express the indicated GFP-GIT1 mutants, or transfected with the HA-GIT1-N fragment alone or together with c-Src. The cells co-transfected with c-Src were treated as in (A,C). Aliquots of the lysates from cells expressing GFP-GIT1 mutants (250 μg of protein) were immunoprecipitated with anti-GFP. Pulldowns: GFP-immunoprecipitates were washed and incubated for 2 h at 4°C with lysates (400 μg of protein) from cells transfected with HA-GIT1-N alone, or together with c-Src. Equal amounts of lysates (25 μg of protein), and the pulldowns were blotted for the detection of the indicated antigens.