Figure 4. Integration of transplanted human NLBs in vivo.
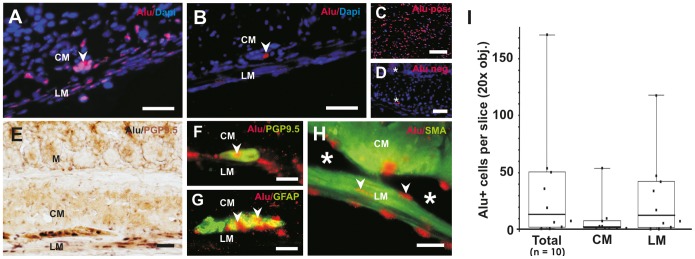
(A) Example of in situ hybridization (ISH) for human-specific Alu sequence with good cell integration within the gut musculature. Note that ganglia-like structures can be detectedat the expected location between the circular and longitudinal muscle layer (arrowhead). (B) Representative example for poor cell integration i.e. only a single cell was detected in this microscopic view (arrowhead). (C) Alu-ISH on human control gut tissue demonstrates the specifity of Alu probe for human cells. (D) ISH with Alu probe on mouse tissue served as negative control. Note, that there is some unspecific background staining in the cytoplasm and extracellular matrix, which does not co-localize with Dapi+ nuclei (stars). (E) Alu-ISH was combined with immunohistochemistry (IHC) to demonstrate in vivo differentiation state of neuronal cells between the longitudinal and circular muscle layers (Alu, black nuclei and PGP9.5, brown cytoplasmic staining). (F–H) Fluorescence ISH for Alu combined with IHC in a higher magnification (Alu-ISH, red nuclei and IHC, green cytoplasma) (F) A PGP9.5/Alu co-stained human cell has been integrated in a small ganglion structure (arrowhead). (G) Differentiated human glial cells within a ganglion as shown by Alu/GFAP-co-staining (arrowheads). (H) Most of the transplanted cells (arrowheads) were found around ganglion structures (stars) in the smooth muscle layers as shown by Alu/SMA-co-staining. (I) Variability of human cell integration was quantified and demonstrated as box-whisker plots. Most cells were found within the longitudinal muscle layer. Scale bars in (A–D) 100 μm, (E) 50 μm, (F–H) 200 μm. M = mucosa, CM = circular muscle, LM = longitudinal muscle.
