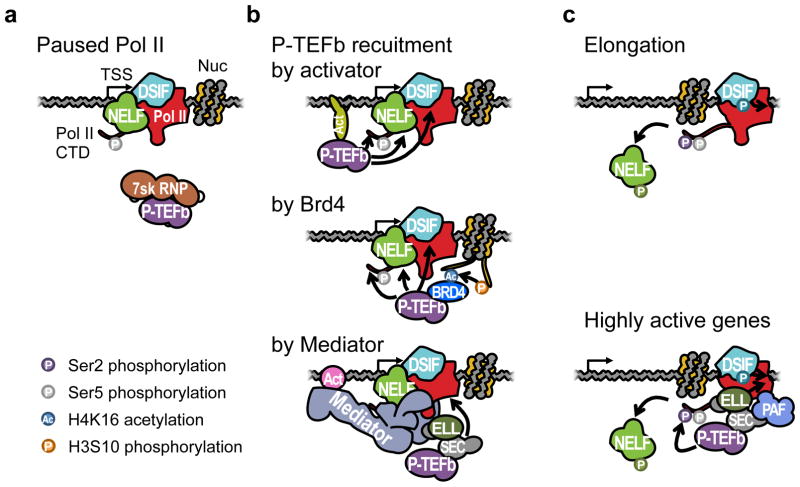
Figure 3. Productive transcription elongation complex.
(a) Paused Pol II is bound by NELF and DSIF. The CTD is phosphorylated at Ser5. P-TEFb is held inactive by 7SK RNP. (b) P-TEFb is activated and recruited to the paused Pol II by various mechanisms. The first is directly by activator that binds to the DNA. Recruited P-TEFb can phosphorylate NELF, DSIF, and Ser2 of Pol II CTD. The second is through Brd4 that binds to acetylated histone tails. In human cells, Brd4 can bind to H4K16 acetylation which is dependent on H3S10 phosphorylation through the ‘histone crosstalk’ (153). The third is indirectly through the Mediator complex, which links the activator (Act) and Pol II. A Mediator subunit MED26 can recruit SEC, which also contains P-TEFb (129). (c) Pol II escapes pausing. Phosphorylated NELF is dissociated from Pol II, and DSIF turns into a positive elongation factor after being phosphorylated by P-TEFb. Alternatively, P-TEFb can remain bound to Pol II by SEC that also interacts with PAF in genes with highly active elongation (76). P-TEFb can continuously phosphorylate Ser2, and a SEC component ELL can facilitate elongation. PAF can also recruit additional elongation factors. RNA is not shown for the clarity of viewing the complexes.