Abstract
Hepatitis C virus, a small single-stranded RNA virus, is a major cause of chronic liver disease. Resolution of primary hepatitis C virus infections depends upon the vigorous responses of CD4+ and CD8+ T cells to multiple viral epitopes. Although such broad CD4+ and CD8+ T-cell responses are readily detected early during the course of infection regardless of clinical outcome, they are not maintained in individuals who develop chronic disease. Purportedly, a variety of factors contribute to the diminished T-cell responses observed in chronic, virus-infected patients including the induction of and biological suppression by CD4+FoxP3+ regulatory T cells. Indeed, a wealth of evidence suggests that regulatory T cells play diverse roles in the pathogenesis of chronic hepatitis C, impairing the effector T-cell response and viral clearance early during the course of infection and suppressing liver injury as the disease progresses. The factors that affect the generation and biological response of regulatory T cells in chronic, hepatitis C virus-infected patients is discussed.
Keywords: dendritic cells, hepatitis C, immune tolerance, liver disease, regulatory T cells
Introduction
Hepatitis C virus (HCV, a small single-stranded RNA virus) is the cause of hepatitis C, the most common blood-borne infection in the world; an estimated 180 million people globally, ~3 million in the US, are chronically infected.1,2 The RNA genome consists of a large open reading frame that encodes an approximate 3,000 amino acid polyprotein precursor that is cleaved by viral and cellular proteases to yield three structural proteins [core, envelope 1 (E1) and E2] and seven non-structural proteins, which are involved in viral synthesis.3 HCV is primarily transmitted via contact with infected blood or bodily fluids. The risk of contracting hepatitis C from contaminated blood or blood products in the US is negligible due to mandatory and reliable blood screening.4 Despite a safe blood supply, however, isolated nosocomial/iatrogenic infections continue to occur emphasizing the need for enforcement of universal precautions and proper sterilization of medical devices.4 Individuals at highest risk of infection in the US today are injection drug users.5 In contrast, a large percentage of the population in many developing countries is infected owing to substandard infrastructure and technology required to provide a safe blood supply.6
The majority of HCV-infected patients develops chronic disease; only an approximate 20% resolves infection spontaneously.2 A variety of factors such as age, sex, ethnicity, human immunodeficiency virus or hepatitis B co-infection, and host genetics (e.g., IL28B gene variants and HLA haplotype) affect spontaneous clearance of HCV.7-10 Standard treatment of unresolved infections, consisting of the combined administration of PEGylated interferon (IFN) and ribavirin over an extended period of 24 to 48 weeks, is often accompanied by significant side effects. Moreover, only 45–50% of treated patients infected with HCV genotype 1 (the primary etiologic agent of hepatitis C in the US) demonstrate a sustained virologic response following treatment withdrawal.11 Additional treatment approaches that combine protease inhibitors, e.g., boceprevir and telaprevir, with ribavirin and PEGylated IFN result in sustained virologic responses in up to 80% of patients infected with HCV genotype 1, though the severity of accompanying side effects can also increase dramatically.12-14
The vigorous responses of HLA class I- (CD8+) and class II-restricted (CD4+) T cells to numerous viral epitopes derived from both structural and nonstructural proteins are required for the resolution of primary HCV infections.15 Such broad responses are readily detected during the early course of infection despite clinical outcome, but subside in patients who later develop chronic disease.16 While patients who spontaneously clear infection continue to exhibit sustained, proliferative responses to a broad array of class I- and class II-restricted epitopes, chronically infected individuals respond to only a limited number.17 Purportedly, multiple factors contribute to the reduction in T-cell responses found in patients with chronic infections, notably, the induction of and biological suppression by CD4+FoxP3+ regulatory T(reg) cells.18-22
Regulatory T Cells
Background
CD4+ regulatory T(reg) cells constitute one of the major mechanisms underlying immunological homeostasis and self-tolerance.23 Treg cells are characterized by the constitutive expression of the transcription factor forkhead box P3 (FoxP3) and interleukin (IL)-2 receptor α chain (CD25) on the cell surface. FoxP3 mutation and Treg cell dysfunction result in fatal immunopathology and autoimmune disease in both mice and men.24-26 Treg cells also moderate effector immune responses to infectious diseases that, if sustained at elevated levels, can lead to serious host tissue and organ damage.27 Although a key factor in the maintenance of immune homeostasis, Treg cells can also suppress effective immune responses to autologous tumors (e.g., hematological malignancies and metastatic melanoma) and promote persistent infections by a wide variety of microbial pathogens.27-29
Treg cell phenotype. Two distinct Treg cell subsets, classically distinguished by site of origin, are described in the literature. Natural (n)Treg cells are generated by high-avidity selection in the thymus; inducible (i)Treg cells, on the other hand, derive from conventional (CD4+CD25-FoxP3-) T cells in the periphery following stimulation.30-32 nTreg cells can induce “infectious tolerance” by converting conventional T cells into iTreg cells via two primary methods: cytokine (IL-10, IL-35 or TGF-β)-dependent and dendritic cell (DC)-mediated, cytokine-independent mechanisms.33,34 Purportedly, nTreg and iTreg cells possess complementary immune functions: prevention of autoimmunity and maintenance of a non-inflammatory environment, respectively.31
Notably, no specific marker defines Treg cells or differentiates nTreg and iTreg cell subsets. While FoxP3 expression is a common attribute of both subsets, conventional human T cells lacking immunosuppressive capacity can also express FoxP3 transiently following activation.32 Moreover, despite the near exclusive expression of CD25 by nTreg cells in naïve mice, CD25 is expressed by a much more heterogeneous T-cell population in humans.32 Recent studies report the high level expression of neuropilin-1 on the surface of nTreg, but not iTreg, cells in mice enabling differentiation and separation of these two subsets.35,36 Activated human FoxP3+ Treg cells that express high suppressive activity are also distinguished by presence of glycoprotein A repetitions predominant (GARP, or LRRC32), a cell surface transmembrane protein that contains leucine-rich repeats.37-40 GARP mRNA is specifically expressed by CD4+CD25hi Treg cells, and is rapidly upregulated following T-cell receptor engagement.37,38 GARP anchors transforming growth factor (TGF)-β to the cell surface conferring increased suppressive activity and the ability to induce infectious tolerance.39 Lastly, cell surface expression of ectonucleotidase, CD39, distinguishes activated, effector memory Treg cells capable of abrogating DC maturation and T cell-dependent cytotoxicity.41
Treg Cell Function
Contact-independent mechanisms
Activated Treg cells are able to suppress the activity of a variety of immune cell types, i.e., both CD8+ and CD4+ T cells, NK cells, NKT cells, B cells, macrophages and DCs.42-46 Multiple mechanisms contribute to this suppressive activity although it is widely believed that nTreg cell-mediated suppression is dependent upon direct, cell–cell contact.46 The synthesis of inhibitory cytokines constitutes a principal contact-independent mechanism by which Treg cells in general suppress Teff cell activity (Fig. 1). Both the soluble and membrane-bound forms of TGF-β, for example, play key roles in inducing and/or maintaining iTreg and nTreg cells, and in suppressing conventional effector T(eff) cell activation.45,47,48 Similarly, IL-10 plays a critical role in suppressing CD4+ Teff cell responses to a variety of pathogens used in animal models, as well as those that contribute to human disease.27
Figure 1. Increases in both the number and function of Treg cells have been implicated in the pathogenesis of chronic hepatitis C. Virus-associated regulatory T cell epitopes, homologous to peptide sequences found in the human plasma proteome, induce nTreg cell activation, conversion of Teff to iTreg cells and infectious tolerance (A). Viral epitopes lacking human homology, which are presented by immature DCs, elicit additional HCV-specific iTreg cells (B). Treg cells inhibit Teff cell function by direct, contact-dependent and -independent mechanisms and by indirect mechanisms that affect DC maturation and/or immunostimulatory activity (C).
The constitutive, high-level expression of CD25 (IL-2 receptor α chain) constitutes an additional contact-independent mechanism underlying Treg cell-mediated suppression. Treg cells produce relatively low levels of IL-2 and, as such, require an exogenous source of IL-2 in order to proliferate and survive.49 As a consequence of the rapid consumption of IL-2 by Treg cells, Teff cell populations are deprived of the cytokine necessary for activation.49 The cell surface expression of CD39 and CD73 ectonucleotidases constitutes another mechanism by which Treg cells disrupt the metabolic activity of Teff cells.50 The activity expressed by these molecules abrogates the proinflammatory response of Teff cells by rapidly degrading extracellular ATP released by neighboring, activated or damaged cells.50 Additionally, adenosine generated as a byproduct of ATP degradation further suppresses Teff cell function by binding A2A receptors expressed on the cell surface and inducing T cell anergy.50-52
Contact-dependent mechanisms
A number of contact-dependent mechanisms also facilitate the ability of Treg cells to suppress Teff cell function. For example, Treg cells can exhibit cytotoxic activity and induce Teff cell apoptosis dependent upon the production of granzyme A, granzyme B and perforin.48,53 In addition, cell-surface galectin-1 appears to contribute to the immunosuppressive activity of Treg cells.54 A member of a highly conserved family of β-galactosidase-binding proteins, galectin-1 inhibits proliferation and promotes apoptosis of activated Teff cells.54
Apart from regulating Teff cell function directly, Treg cells can inhibit the maturation and immunostimulatory activity of DC and, consequently, suppress the activity of conventional T cells indirectly.48 Treg cells can render DCs tolerogenic and limit their capacity to sensitize naïve T cells by a process termed trans-endocytosis whereby cytotoxic T lymphocyte-4 (CTLA-4) molecules expressed by Treg cells capture, internalize, and degrade co-stimulatory molecules (i.e., CD80 and CD86) expressed on the surface of DCs.55 Lymphocyte activation gene-3 (LAG-3 of CD223), a CD4 homolog capable of binding MHC class II molecules, further suppresses DC maturation and the expression of co-stimulatory molecules.56,57 Lastly, neuropilin-1 expressed by nTreg cells inhibits the sensitization of naïve CD4+ T cells by promoting extended interaction between Treg cells and immature DCs.58 Undoubtedly, the suppressive functions of Treg cells depend upon a combination of the mechanisms described.
Treg Cells in Hepatitis C
The increased numbers of Treg cells present in the liver and peripheral blood of chronic HCV-infected patients relative to patients who spontaneously clear infection suggests that Treg cells play a significant role in the pathogenesis of chronic hepatitis C.18,19,27,59-63 In this regard, CD4+CD25+ T cells obtained from the peripheral blood of patients infected with HCV suppressed virus-specific proliferation and IFN-γ production by CD4+ and CD8+ T cell; depletion of CD25+ cells including Tregs from the total peripheral blood mononuclear cell (PBMC) population enhanced the proliferation of cells that remained.18,63 Whether the increase number of Treg cells found in the livers of chronically-infected patients represents an virus-specific Treg cell response or arises as a non-specific consequence of chronic inflammation and disease is not entirely clear.
HCV encodes Treg cell epitopes
Researchers have identified Treg cell epitopes present in both the structural and non-structural proteins synthesized by HCV.20,59,60,64-66 Using HCV peptide-loaded MHC class II tetramers to label and quantify the cells, Ebimuna and coworkers reported HCV epitope-specific recognition by FoxP3+ Treg cells present in the peripheral blood of chronically infected patients.64 Moreover, a number of studies now report an increase in FoxP3+CD25high cells that resemble nTreg cells and express an anergic cytokine profile following stimulation with HCV derived epitopes.59,61,64 Comparing FACS-sorted CD4+CD25high T cells (nTreg phenotype) derived from uninfected and HCV-infected individuals, however, one study found only minimal differences in global gene expression.64 In contrast, other investigators reported an increase in HCV epitope-specific Treg cells that resembled iTreg cells phenotypically and exhibited both IL-10- and TGF-β-dependent suppressive activity.60,65 Regardless, a general consensus supports the heterogeneous nature of the expanded Treg cell population found in chronic, HCV-infected patients (i.e., composed of both nTreg and iTreg cell subsets). The factors that contribute to this expansion are neither well understood nor characterized. Conceivably, the initial response of nTreg cells to infection promotes the conversion of conventional T cells to iTreg cells and subsequent suppression (i.e., infectious tolerance).34
HCV Treg cell epitopes and human homology
The expressed function of nTreg cells is to suppress autoimmunity and the response to self antigens.23-26 Activation of Treg cells subsequent to HCV infection suggests the existence of viral epitopes that exhibit homology to self and the ability to simulate these nTreg cells and viral persistence.59,67-70 Indeed, Kanduc and colleagues reported that 3003 unique pentapeptides comprised the HCV polyprotein; of these, 2760 pentapeptides occurred a total of 46,731 times (including matches/repeats) in 20,269 proteins that comprised the human proteome.68,70 Furthermore, a BLAST search and JanusMatrix analysis (a new computer algorithm, which identifies epitopes that are highly cross-reactive on the T cell receptor aspect) revealed extensive homology between a number of published Treg cell epitopes derived from HCV structural and non-structural proteins and sequences encoded by the human genome (Moise et al., manuscript submitted) (Losikoff et al., manuscript in preparation).71 These epitopes induced the response of Treg cells present in the peripheral blood of chronic HCV-infected patients, but not in blood obtained from HCV non-infected individuals.22,59,71 Taken together, these findings suggest that viral epitopes, homologous to peptide sequences constituting the human proteome, influence the pathogenesis of chronic hepatitis C by inducing the expansion of the iTreg cell population and infectious tolerance.62,72
An evolving body of research demonstrates the import of the human microbiome in shaping the immune response to viral pathogens.73 Recent studies found the microbiome can induce the differentiation and expansion of iTreg cells that exhibit a unique T cell receptor repertoire specific for commensal bacteria.74,75 Conceivably, homology between viral epitopes and Treg cell epitopes associated with the human microbiome represents an additional mechanism underlying the expansion of Treg cells in chronic HCV.
Treg Cells Impair Viral Clearance
Several cross-sectional studies demonstrated a strong correlation between chronic HCV infection and increased Treg cell frequencies.18,63,64 The specific contribution of Treg cells to viral persistence, however, could not be elucidated due to the occurrence of infection at some previous, undetermined date. Furthermore, two longitudinal studies failed to demonstrate a significant difference in the frequency or function of Treg cells in the blood of patients acutely infected with HCV regardless of clinical outcome (i.e., whether the virus was eventually cleared or chronic disease developed).61,76 In one of these studies, Treg cells obtained from patients who subsequently developed chronic disease demonstrated significantly more suppressive activity than did Treg cells obtained from patients who spontaneously cleared infection.61 As a consequence, the authors concluded that maintenance of an elevated Treg cell population promoted the development of chronic hepatitis C.
Recently, Cusick and coworkers described the emergence of a variant of an immunodominant HCV epitope during the course of chronic HCV infection.66 This variant elicited a Treg cell population that suppressed a Teff cell response to the cognate, but not an unrelated, peptide. This finding suggests that variant, MHC class II-restricted viral epitopes arise as a consequence of immune pressure during the course of chronic HCV infection. Rather than induce the activity of conventional CD4+ T cells, these variant epitopes elicit an epitope-specific Treg cells response that diminishes the CD4+ T cell help required for viral clearance. Thus, in cases of chronic disease, Treg cells impair Teff cell function and promote viral persistence by inhibiting virus-specific helper T cell activity.
Treg Cells Suppress Chronic Hepatitis C-Associated Liver Injury
In addition to inhibiting effective immune responses and promoting persistent infections by a wide variety of pathogenic microorganisms, Treg cells moderate Teff cell responses that could otherwise lead to serious host tissue and organ damage.18,21,27,28,77-79 This is evidenced in patients with chronic hepatitis C by an inverse correlation between the level of Treg cell activity found in the peripheral blood and the extent of liver injury.18,78,80 Both the serum alanine aminotransferase concentrations and the histopathologic scores of liver biopsies were higher in chronic HCV-infected patients who exhibited a relative reduction in Treg cell activity suggesting that the expanded Treg cell population ameliorated excessive pathology and tissue damage. The balance between Treg cells and CD8+ Teff cells appears to be a crucial factor. Sturm and colleagues reported a strong inverse correlation between the severity of liver fibrosis and the accumulation of CD4+FoxP3+ Treg cells in close contact with CD8+ T cells in necro-inflammatory sites in patients with chronic hepatitis C.21 The ratio of FoxP3+ to CD8+ T cells was elevated in the early stages of fibrosis, and reduced substantially in cirrhotic livers. The authors also reported a link between IL-10 and TGF-β message expression in the liver and presence of FoxP3+ and CD8+ T cells suggesting that these anti-inflammatory cytokines served both to maintain the Treg cell pool and inhibit the production of pro-inflammatory cytokine (e.g., TNF-α and IFN-γ) by CD8 Teff cells.21
Thus, an equilibrium between Treg cells and Teff cells that permits both viral clearance and protection from HCV-related hepatopathology is critical for an optimal response to HCV infection.21,77 In cases of chronic disease, however, the elevated presence of Treg cells appears to benefit both the pathogen (i.e., viral persistence) and the host (i.e., prevention of immunopathogenesis).21,79 In this regard, it is Interesting to note that increased numbers of CD25+FoxP3+ Treg cells were found in the livers of patients who underwent successful chemotherapy, suggesting that the continued presence of Treg cells moderated immunopathology despite viral clearance. These divergent roles of Treg cells during the development of chronic hepatitis C are illustrated in Figure 2.
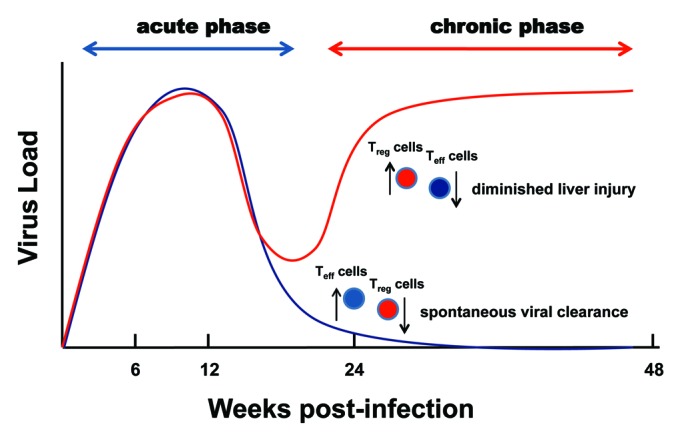
Figure 2. Contrasting contributions of Treg cells to the pathogenesis of chronic hepatitis C. An increased ratio of Treg to Teff cells impairs spontaneous viral clearance and suppresses liver injury and the pathogenesis of chronic hepatitis C as the disease progresses.
Impaired DC Function Promotes the Generation of Treg Cells
Whether HCV infection elicits the response of Treg cells directly or indirectly (dependent upon the intermediary role of another cell type) remains to be determined. Accumulating evidence suggests, however, that DCs play a key role in the expansion of Treg cells during the pathogenesis of chronic disease. DCs are professional antigen presenting cells characterized by the potent capacity to elicit primary T cell responses.81 While there is no general consensus regarding the effects of HCV infection on DC function, it is often agreed that chronically infected patients have significantly fewer DCs circulating in the peripheral blood, which correlates inversely with the severity of liver disease.82-86 Conversely, increased numbers of DCs are found in the liver of patients chronically infected with HCV.85,87-89 Regardless of their physiologic distribution (i.e., peripheral blood vs. liver), it is frequently suggested that the DCs are functionally impaired in patients with chronic hepatitis C.83,89-98 These impairments include lowered expression of HLA-DR and costimulatory molecules (e.g., CD86), decreased allostimulatory activity and the ability to secrete IFN-α and IL-12 and increased IL-10 production and the ability to prime Treg cells.83-85,89,90,92-96,99-103 Additionally, it was reported that DCs derived from chronically infected patients remain phenotypically immature and lack the capacity to upregulate the expression of maturation or costimulatory marker following stimulation.91,97
Mechanisms that underlie impaired DC function
The specific mechanisms underlying DC dysfunction in patients chronically infected with HCV remain to be clarified. Recent studies suggest, on the one hand, that DCs are susceptible to HCV infection.104,105 The interaction between envelope glycoproteins E1 and E2 and DC-SIGN, a C-type lectin expressed by DCs, mediates the ingestion of HCV viral particles.105 This finding supports the speculation that, even in the absence of a productive infection, E1 and E2 bound to DC-SIGN transmit a signal that promotes Treg cell formation and tolerance. Other structural and non-structural viral proteins further impair DC function by decreasing costimulatory molecule and HLA expression, reducing cytokine production, preventing TLR signaling and diminishing allostimulatory activity.106 As described above, Treg cells can also inhibit DC maturation and immunostimulatory capacity by a variety of mechanisms and, thereby, suppress the response of conventional T cells.48 A growing body of evidence supports the critical role of DCs in the induction and maintenance of Treg cells during chronic HCV infection, regardless of the specific mechanisms that contribute to a diminution in DC function.
DCs and immunological tolerance
DCs play a key role in establishing and maintaining immunological tolerance to both foreign and self antigens.107,108 Interactions between Treg cells and DCs in the prototypic, tolerogenic environment maintained in the liver foster the development of chronic infectious diseases such as viral hepatitis.109 While it is generally agreed that immature DCs promote Treg cell function, the underlying mechanisms remain to be fully delineated.107-111 Notably, DCs obtained from human liver principally exhibit an immature phenotype characterized by low or negligible expression of cell-surface co-stimulatory molecule (e.g., CD40, CD80, CD83 and CD86).109 Relative to DCs purified from the peripheral blood, DCs derived from liver also produce lower levels of proinflammatory cytokine (i.e., TNF-α, IL-1β and IL-6) and significantly higher levels of IL-10 following stimulation. Moreover, the IL-10-dependent production of CD4+CD25+FoxP3+ Treg cells was greater in co-cultures that contained naïve, allogeneic CD4+ T cells and liver DCs, compared with co-cultures that contained DCs derived from blood.110 Similar results were obtained when allogeneic T cells and DCs obtained from chronic, HCV-infected patients were co-cultured, i.e., greater expansion of the Treg cell population than observed in co-cultures that contained DCs obtained from healthy individuals.103
Summary and Clinical Implications
Eighty to 85% of the patients in the US infected with HCV develop chronic disease.2 Persistent viral infection correlates with increased Treg cell number and function in the tissues of HCV-infected patients. While triple drug therapy (e.g., boceprevir or telapervir administered in conjunction with interferon and ribavirin) increases the sustained virologic response, the adverse side effects are often severe, the costs are extraordinarily high, and a significant portion of treated patients remains infected.12-14 Additional approaches, e.g., therapeutic vaccination, are urgently needed. To date, four vaccine strategies have demonstrated varied and only limited success in clinical trials: recombinant protein, peptide, genetic or DNA-based and vector-mediated.112,113 Recent evidence suggests that viral epitopes may activate nTreg cells and, subsequently, induce expansion of the iTreg cell population and immunosuppression via the direct effects of Treg cells on Teff cell activity or by indirect effects dependent upon intervening DCs. DCs that exhibit an immature phenotype, in turn, can promote the expansion and activity of Treg cells and, thus, play a key role in exacerbating the pathogenesis of chronic hepatitis C. As a consequence, future efforts to develop a therapeutic vaccine must employ strategies to avoid incorporating epitopes that promote Treg cell activity.
In the absence of a therapeutic vaccine, a full understanding of the response of Treg cells to HCV infection could lead to the development of treatments capable of sustaining Teff cell-mediated immunity while limiting tissue damage.114 In this regard, continued research efforts are needed to determine the most effective means of manipulating the Treg cell response in a clinical setting.18 Progress in understanding the role of Treg cells and the factors that influence protective immunity to hepatitis C are hampered, however, by the lack of a good rodent model and the ability of HCV to infect humans and chimpanzees only.115
References
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Support was provided by National Institutes of Health Research Grant: U19 AI082642.
Glossary
Abbreviations:
- DCs
dendritic cells
- FoxP3
transcription factor forkhead box P3
- HCV
hepatitis C virus
- IFN
interferon
- IL
interleukin
- iTreg cell
inducible regulatory T cell
- nTreg cell
natural regulatory T cell
- Teff cells
effector T cells
- TGF-β
transforming growth factor-beta
Footnotes
Previously published online: www.landesbioscience.com/journals/vaccines/article/24726
References
- 1.Alter MJ, Margolis HS, Krawczynski K, Judson FN, Mares A, Alexander WJ, et al. The natural history of community-acquired hepatitis C in the United States. The Sentinel Counties Chronic non-A, non-B Hepatitis Study Team. N Engl J Med. 1992;327:1899–905. doi: 10.1056/NEJM199212313272702. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma and hepatitis C in the United States. Hepatology. 2002;36(Suppl 1):S74–83. doi: 10.1002/hep.1840360710. [DOI] [PubMed] [Google Scholar]
- 3.Grakoui A, Wychowski C, Lin C, Feinstone SM, Rice CM. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993;67:1385–95. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Healthcare-associated hepatitis B and C outbreaks reported to the Centers for Disease Control and Prevention (CDC) in 2008-2011. CDC Website 2012.
- 5.Amon JJ, Garfein RS, Ahdieh-Grant L, Armstrong GL, Ouellet LJ, Latka MH, et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994-2004. Clin Infect Dis. 2008;46:1852–8. doi: 10.1086/588297. [DOI] [PubMed] [Google Scholar]
- 6.Brown RS, Jr., Gaglio PJ. Scope of worldwide hepatitis C problem. Liver Transpl. 2003;9:S10–3. doi: 10.1053/jlts.2003.50244. [DOI] [PubMed] [Google Scholar]
- 7.Jauncey M, Micallef JM, Gilmour S, Amin J, White PA, Rawlinson W, et al. Clearance of hepatitis C virus after newly acquired infection in injection drug users. J Infect Dis. 2004;190:1270–4. doi: 10.1086/423943. [DOI] [PubMed] [Google Scholar]
- 8.Rauch A, Gaudieri S, Thio C, Bochud PY. Host genetic determinants of spontaneous hepatitis C clearance. Pharmacogenomics. 2009;10:1819–37. doi: 10.2217/pgs.09.121. [DOI] [PubMed] [Google Scholar]
- 9.Kuzushita N, Hayashi N, Moribe T, Katayama K, Kanto T, Nakatani S, et al. Influence of HLA haplotypes on the clinical courses of individuals infected with hepatitis C virus. Hepatology. 1998;27:240–4. doi: 10.1002/hep.510270136. [DOI] [PubMed] [Google Scholar]
- 10.Neumann-Haefelin C. [Protective role of HLA-B27 in HIV and hepatitis C virus infection] Dtsch Med Wochenschr. 2011;136:320–4. doi: 10.1055/s-0031-1272531. [DOI] [PubMed] [Google Scholar]
- 11.Davis GL, Esteban-Mur R, Rustgi V, Hoefs J, Gordon SC, Trepo C, et al. International Hepatitis Interventional Therapy Group Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N Engl J Med. 1998;339:1493–9. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 12.Schaefer EA, Chung RT. Anti-hepatitis C virus drugs in development. Gastroenterology. 2012;142:1340–50, e1. doi: 10.1053/j.gastro.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 13.U.S.Food and Drug Administration. Incivek (telaprevir) in combination with drugs peginterferon alfa and ribavirin (incivek combination treatment): drug safety communication - serious skin reactions. Electronic Communication, USDA Website 2012.
- 14.Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156:279–90. doi: 10.7326/0003-4819-156-4-201202210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lechner F, Wong DK, Dunbar PR, Chapman R, Chung RT, Dohrenwend P, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulze Zur Wiesch J, Ciuffreda D, Lewis-Ximenez L, Kasprowicz V, Nolan BE, Streeck H, et al. Broadly directed virus-specific CD4+ T cell responses are primed during acute hepatitis C infection, but rapidly disappear from human blood with viral persistence. J Exp Med. 2012;209:61–75. doi: 10.1084/jem.20100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day CL, Lauer GM, Robbins GK, McGovern B, Wurcel AG, Gandhi RT, et al. Broad specificity of virus-specific CD4+ T-helper-cell responses in resolved hepatitis C virus infection. J Virol. 2002;76:12584–95. doi: 10.1128/JVI.76.24.12584-12595.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabrera R, Tu Z, Xu Y, Firpi RJ, Rosen HR, Liu C, et al. An immunomodulatory role for CD4(+)CD25(+) regulatory T lymphocytes in hepatitis C virus infection. Hepatology. 2004;40:1062–71. doi: 10.1002/hep.20454. [DOI] [PubMed] [Google Scholar]
- 19.Ward SM, Fox BC, Brown PJ, Worthington J, Fox SB, Chapman RW, et al. Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J Hepatol. 2007;47:316–24. doi: 10.1016/j.jhep.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 20.Li S, Floess S, Hamann A, Gaudieri S, Lucas A, Hellard M, et al. Analysis of FOXP3+ regulatory T cells that display apparent viral antigen specificity during chronic hepatitis C virus infection. PLoS Pathog. 2009;5:e1000707. doi: 10.1371/journal.ppat.1000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sturm N, Thélu MA, Camous X, Dimitrov G, Ramzan M, Dufeu-Duchesne T, et al. Characterization and role of intra-hepatic regulatory T cells in chronic hepatitis C pathogenesis. J Hepatol. 2010;53:25–35. doi: 10.1016/j.jhep.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Gowans EJ, Chougnet C, Plebanski M, Dittmer U. Natural regulatory T cells and persistent viral infection. J Virol. 2008;82:21–30. doi: 10.1128/JVI.01768-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 24.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. Targeted disruption of the mouse transforming growth factor-β 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–9. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–1. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 26.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–42. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 27.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu Rev Immunol. 2009;27:551–89. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 28.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–44. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–46. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 31.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–35. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Miyara M, Sakaguchi S. Human FoxP3(+)CD4(+) regulatory T cells: their knowns and unknowns. Immunol Cell Biol. 2011;89:346–51. doi: 10.1038/icb.2010.137. [DOI] [PubMed] [Google Scholar]
- 33.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196:255–60. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gravano DM, Vignali DA. The battle against immunopathology: infectious tolerance mediated by regulatory T cells. Cell Mol Life Sci. 2012;69:1997–2008. doi: 10.1007/s00018-011-0907-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–22, S1-19. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss JM, Bilate AM, Gobert M, Ding Y, Curotto de Lafaille MA, Parkhurst CN, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med 2012;209:1723-42, S1. [DOI] [PMC free article] [PubMed]
- 37.Wang R, Wan Q, Kozhaya L, Fujii H, Unutmaz D. Identification of a regulatory T cell specific cell surface molecule that mediates suppressive signals and induces Foxp3 expression. PLoS One. 2008;3:e2705. doi: 10.1371/journal.pone.0002705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13439–44. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:13445–50. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stockis J, Colau D, Coulie PG, Lucas S. Membrane protein GARP is a receptor for latent TGF-beta on the surface of activated human Treg. Eur J Immunol. 2009;39:3315–22. doi: 10.1002/eji.200939684. [DOI] [PubMed] [Google Scholar]
- 41.Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10:2410–20. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–32. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghiringhelli F, Ménard C, Martin F, Zitvogel L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol Rev. 2006;214:229–38. doi: 10.1111/j.1600-065X.2006.00445.x. [DOI] [PubMed] [Google Scholar]
- 44.La Cava A, Van Kaer L, Fu-Dong-Shi CD4+CD25+ Tregs and NKT cells: regulators regulating regulators. Trends Immunol. 2006;27:322–7. doi: 10.1016/j.it.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–45. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–16. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey H, O’Shea JJ, et al. CD4+ FoxP3+ regulatory T cells confer infectious tolerance in a TGF-beta-dependent manner. J Exp Med. 2008;205:1975–81. doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–32. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaguchi T, Wing JB, Sakaguchi S. Two modes of immune suppression by Foxp3(+) regulatory T cells under inflammatory or non-inflammatory conditions. Semin Immunol. 2011;23:424–30. doi: 10.1016/j.smim.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225–32. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 51.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–65. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, et al. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23:1685–93. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ren X, Ye F, Jiang Z, Chu Y, Xiong S, Wang Y. Involvement of cellular death in TRAIL/DR5-dependent suppression induced by CD4(+)CD25(+) regulatory T cells. Cell Death Differ. 2007;14:2076–84. doi: 10.1038/sj.cdd.4402220. [DOI] [PubMed] [Google Scholar]
- 54.Garín MI, Chu CC, Golshayan D, Cernuda-Morollón E, Wait R, Lechler RI. Galectin-1: a key effector of regulation mediated by CD4+CD25+ T cells. Blood. 2007;109:2058–65. doi: 10.1182/blood-2006-04-016451. [DOI] [PubMed] [Google Scholar]
- 55.Qureshi OS, Zheng Y, Nakamura K, Attridge K, Manzotti C, Schmidt EM, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science. 2011;332:600–3. doi: 10.1126/science.1202947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–13. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 57.Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. J Immunol. 2008;180:5916–26. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- 58.Sarris M, Andersen KG, Randow F, Mayr L, Betz AG. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–13. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li S, Jones KL, Woollard DJ, Dromey J, Paukovics G, Plebanski M, et al. Defining target antigens for CD25+ FOXP3 + IFN-γ- regulatory T cells in chronic hepatitis C virus infection. Immunol Cell Biol. 2007;85:197–204. doi: 10.1038/sj.icb.7100020. [DOI] [PubMed] [Google Scholar]
- 60.MacDonald AJ, Duffy M, Brady MT, McKiernan S, Hall W, Hegarty J, et al. CD4 T helper type 1 and regulatory T cells induced against the same epitopes on the core protein in hepatitis C virus-infected persons. J Infect Dis. 2002;185:720–7. doi: 10.1086/339340. [DOI] [PubMed] [Google Scholar]
- 61.Smyk-Pearson S, Golden-Mason L, Klarquist J, Burton JR, Jr., Tester IA, Wang CC, et al. Functional suppression by FoxP3+CD4+CD25(high) regulatory T cells during acute hepatitis C virus infection. J Infect Dis. 2008;197:46–57. doi: 10.1086/523651. [DOI] [PubMed] [Google Scholar]
- 62.Boettler T, Spangenberg HC, Neumann-Haefelin C, Panther E, Urbani S, Ferrari C, et al. T cells with a CD4+CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J Virol. 2005;79:7860–7. doi: 10.1128/JVI.79.12.7860-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sugimoto K, Ikeda F, Stadanlick J, Nunes FA, Alter HJ, Chang KM. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex vivo in persistent HCV infection. Hepatology. 2003;38:1437–48. doi: 10.1016/j.hep.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 64.Ebinuma H, Nakamoto N, Li Y, Price DA, Gostick E, Levine BL, et al. Identification and in vitro expansion of functional antigen-specific CD25+ FoxP3+ regulatory T cells in hepatitis C virus infection. J Virol. 2008;82:5043–53. doi: 10.1128/JVI.01548-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Langhans B, Braunschweiger I, Arndt S, Schulte W, Satoguina J, Layland LE, et al. Core-specific adaptive regulatory T-cells in different outcomes of hepatitis C. Clin Sci (Lond) 2010;119:97–109. doi: 10.1042/CS20090661. [DOI] [PubMed] [Google Scholar]
- 66.Cusick MF, Schiller JJ, Gill JC, Eckels DD. Hepatitis C virus induces regulatory T cells by naturally occurring viral variants to suppress T cell responses. Clin Dev Immunol 2011;2011:806061. [DOI] [PMC free article] [PubMed]
- 67.Mishiro S, Takeda K, Hoshi Y, Yoshikawa A, Gotanda T, Itoh Y. An autoantibody cross-reactive to hepatitis C virus core and a host nuclear antigen. Autoimmunity. 1991;10:269–73. doi: 10.3109/08916939109001900. [DOI] [PubMed] [Google Scholar]
- 68.Kanduc D, Stufano A, Lucchese G, Kusalik A. Massive peptide sharing between viral and human proteomes. Peptides. 2008;29:1755–66. doi: 10.1016/j.peptides.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kanduc D. HCV: Written in our DNA. Self Nonself. 2011;2:108–13. doi: 10.4161/self.2.2.15795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kusalik A, Bickis M, Lewis C, Li Y, Lucchese G, Marincola FM, et al. Widespread and ample peptide overlapping between HCV and Homo sapiens proteomes. Peptides. 2007;28:1260–7. doi: 10.1016/j.peptides.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 71.Losikoff P, Terry F, Mishra S, Martin W, Ardito M, Bailey-Kellogg C, et al. A hepatitis C virus-encoded epitope, homologous to a wide array of predicted human epitopes, stimulates a T-regulatory cell response in patients with chronic hepatitis C. New England Regional Center of Excellence Annual Retreat 2012 2012.
- 72.Rushbrook SM, Ward SM, Unitt E, Vowler SL, Lucas M, Klenerman P, et al. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J Virol. 2005;79:7852–9. doi: 10.1128/JVI.79.12.7852-7859.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pfeiffer JK, Sonnenburg JL. The intestinal microbiota and viral susceptibility. Front Microbiol. 2011;2:92. doi: 10.3389/fmicb.2011.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio CW, Santacruz N, et al. Peripheral education of the immune system by colonic commensal microbiota. Nature. 2011;478:250–4. doi: 10.1038/nature10434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heeg MH, Ulsenheimer A, Grüner NH, Zachoval R, Jung MC, Gerlach JT, et al. FOXP3 expression in hepatitis C virus-specific CD4+ T cells during acute hepatitis C. Gastroenterology. 2009;137:1280–8, e1-6. doi: 10.1053/j.gastro.2009.06.059. [DOI] [PubMed] [Google Scholar]
- 77.Manigold T, Racanelli V. T-cell regulation by CD4 regulatory T cells during hepatitis B and C virus infections: facts and controversies. Lancet Infect Dis. 2007;7:804–13. doi: 10.1016/S1473-3099(07)70289-X. [DOI] [PubMed] [Google Scholar]
- 78.Speletas M, Argentou N, Germanidis G, Vasiliadis T, Mantzoukis K, Patsiaoura K, et al. Foxp3 expression in liver correlates with the degree but not the cause of inflammation. Mediators Inflamm 2011;2011:827565. [DOI] [PMC free article] [PubMed]
- 79.Rouse BT, Suvas S. Regulatory cells and infectious agents: detentes cordiale and contraire. J Immunol. 2004;173:2211–5. doi: 10.4049/jimmunol.173.4.2211. [DOI] [PubMed] [Google Scholar]
- 80.Bolacchi F, Sinistro A, Ciaprini C, Demin F, Capozzi M, Carducci FC, et al. Increased hepatitis C virus (HCV)-specific CD4+CD25+ regulatory T lymphocytes and reduced HCV-specific CD4+ T cell response in HCV-infected patients with normal versus abnormal alanine aminotransferase levels. Clin Exp Immunol. 2006;144:188–96. doi: 10.1111/j.1365-2249.2006.03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 82.Ryan EJ, O’Farrelly C. The affect of chronic hepatitis C infection on dendritic cell function: a summary of the experimental evidence. J Viral Hepat. 2011;18:601–7. doi: 10.1111/j.1365-2893.2011.01453.x. [DOI] [PubMed] [Google Scholar]
- 83.Ulsenheimer A, Gerlach JT, Jung MC, Gruener N, Wächtler M, Backmund M, et al. Plasmacytoid dendritic cells in acute and chronic hepatitis C virus infection. Hepatology. 2005;41:643–51. doi: 10.1002/hep.20592. [DOI] [PubMed] [Google Scholar]
- 84.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–54. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cicinnati VR, Kang J, Sotiropoulos GC, Hilgard P, Frilling A, Broelsch CE, et al. Altered chemotactic response of myeloid and plasmacytoid dendritic cells from patients with chronic hepatitis C: role of alpha interferon. J Gen Virol. 2008;89:1243–53. doi: 10.1099/vir.0.83517-0. [DOI] [PubMed] [Google Scholar]
- 86.Kunitani H, Shimizu Y, Murata H, Higuchi K, Watanabe A. Phenotypic analysis of circulating and intrahepatic dendritic cell subsets in patients with chronic liver diseases. J Hepatol. 2002;36:734–41. doi: 10.1016/S0168-8278(02)00062-4. [DOI] [PubMed] [Google Scholar]
- 87.Nattermann J, Zimmermann H, Iwan A, von Lilienfeld-Toal M, Leifeld L, Nischalke HD, et al. Hepatitis C virus E2 and CD81 interaction may be associated with altered trafficking of dendritic cells in chronic hepatitis C. Hepatology. 2006;44:945–54. doi: 10.1002/hep.21350. [DOI] [PubMed] [Google Scholar]
- 88.Wald O, Weiss ID, Galun E, Peled A. Chemokines in hepatitis C virus infection: pathogenesis, prognosis and therapeutics. Cytokine. 2007;39:50–62. doi: 10.1016/j.cyto.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 89.Wertheimer AM, Bakke A, Rosen HR. Direct enumeration and functional assessment of circulating dendritic cells in patients with liver disease. Hepatology. 2004;40:335–45. doi: 10.1002/hep.20306. [DOI] [PubMed] [Google Scholar]
- 90.Kanto T, Hayashi N, Takehara T, Tatsumi T, Kuzushita N, Ito A, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–91. [PubMed] [Google Scholar]
- 91.Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171–6. doi: 10.1182/blood.V97.10.3171. [DOI] [PubMed] [Google Scholar]
- 92.Anthony DD, Yonkers NL, Post AB, Asaad R, Heinzel FP, Lederman MM, et al. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J Immunol. 2004;172:4907–16. doi: 10.4049/jimmunol.172.8.4907. [DOI] [PubMed] [Google Scholar]
- 93.Murakami H, Akbar SM, Matsui H, Horiike N, Onji M. Decreased interferon-alpha production and impaired T helper 1 polarization by dendritic cells from patients with chronic hepatitis C. Clin Exp Immunol. 2004;137:559–65. doi: 10.1111/j.1365-2249.2004.02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Szabo G, Dolganiuc A. Subversion of plasmacytoid and myeloid dendritic cell functions in chronic HCV infection. Immunobiology. 2005;210:237–47. doi: 10.1016/j.imbio.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 95.Della Bella S, Crosignani A, Riva A, Presicce P, Benetti A, Longhi R, et al. Decrease and dysfunction of dendritic cells correlate with impaired hepatitis C virus-specific CD4+ T-cell proliferation in patients with hepatitis C virus infection. Immunology. 2007;121:283–92. doi: 10.1111/j.1365-2567.2007.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Averill L, Lee WM, Karandikar NJ. Differential dysfunction in dendritic cell subsets during chronic HCV infection. Clin Immunol. 2007;123:40–9. doi: 10.1016/j.clim.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gelderblom HC, Nijhuis LE, de Jong EC, te Velde AA, Pajkrt D, Reesink HW, et al. Monocyte-derived dendritic cells from chronic HCV patients are not infected but show an immature phenotype and aberrant cytokine profile. Liver Int. 2007;27:944–53. doi: 10.1111/j.1478-3231.2007.01507.x. [DOI] [PubMed] [Google Scholar]
- 98.MacDonald AJ, Semper AE, Libri NA, Rosenberg WM. Monocyte-derived dendritic cell function in chronic hepatitis C is impaired at physiological numbers of dendritic cells. Clin Exp Immunol. 2007;148:494–500. doi: 10.1111/j.1365-2249.2007.03367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kanto T, Inoue M, Miyatake H, Sato A, Sakakibara M, Yakushijin T, et al. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190:1919–26. doi: 10.1086/425425. [DOI] [PubMed] [Google Scholar]
- 100.Saito K, Ait-Goughoulte M, Truscott SM, Meyer K, Blazevic A, Abate G, et al. Hepatitis C virus inhibits cell surface expression of HLA-DR, prevents dendritic cell maturation, and induces interleukin-10 production. J Virol. 2008;82:3320–8. doi: 10.1128/JVI.02547-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mengshol JA, Golden-Mason L, Castelblanco N, Im KA, Dillon SM, Wilson CC, et al. Virahep-C Study Group Impaired plasmacytoid dendritic cell maturation and differential chemotaxis in chronic hepatitis C virus: associations with antiviral treatment outcomes. Gut. 2009;58:964–73. doi: 10.1136/gut.2008.168948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bain C, Fatmi A, Zoulim F, Zarski JP, Trépo C, Inchauspé G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–24. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 103.Dolganiuc A, Paek E, Kodys K, Thomas J, Szabo G. Myeloid dendritic cells of patients with chronic HCV infection induce proliferation of regulatory T lymphocytes. Gastroenterology. 2008;135:2119–27. doi: 10.1053/j.gastro.2008.07.082. [DOI] [PubMed] [Google Scholar]
- 104.Blackard JT, Smeaton L, Hiasa Y, Horiike N, Onji M, Jamieson DJ, et al. Detection of hepatitis C virus (HCV) in serum and peripheral-blood mononuclear cells from HCV-monoinfected and HIV/HCV-coinfected persons. J Infect Dis. 2005;192:258–65. doi: 10.1086/430949. [DOI] [PubMed] [Google Scholar]
- 105.Ludwig IS, Lekkerkerker AN, Depla E, Bosman F, Musters RJ, Depraetere S, et al. Hepatitis C virus targets DC-SIGN and L-SIGN to escape lysosomal degradation. J Virol. 2004;78:8322–32. doi: 10.1128/JVI.78.15.8322-8332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krishnadas DK, Ahn JS, Han J, Kumar R, Agrawal B. Immunomodulation by hepatitis C virus-derived proteins: targeting human dendritic cells by multiple mechanisms. Int Immunol. 2010;22:491–502. doi: 10.1093/intimm/dxq033. [DOI] [PubMed] [Google Scholar]
- 107.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 108.Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–65. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 110.Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, et al. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol. 2009;182:1901–11. doi: 10.4049/jimmunol.0803404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cobbold SP, Adams E, Nolan KF, Regateiro FS, Waldmann H. Connecting the mechanisms of T-cell regulation: dendritic cells as the missing link. Immunol Rev. 2010;236:203–18. doi: 10.1111/j.1600-065X.2010.00913.x. [DOI] [PubMed] [Google Scholar]
- 112.Yu CI, Chiang BL. A new insight into hepatitis C vaccine development. J Biomed Biotechnol 2010;2010:548280. [DOI] [PMC free article] [PubMed]
- 113.Halliday J, Klenerman P, Barnes E. Vaccination for hepatitis C virus: closing in on an evasive target. Expert Rev Vaccines. 2011;10:659–72. doi: 10.1586/erv.11.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Keynan Y, Card CM, McLaren PJ, Dawood MR, Kasper K, Fowke KR. The role of regulatory T cells in chronic and acute viral infections. Clin Infect Dis. 2008;46:1046–52. doi: 10.1086/529379. [DOI] [PubMed] [Google Scholar]
- 115.Billerbeck E, de Jong Y, Dorner M, de la Fuente C, Ploss A. Animal models for hepatitis C. Curr Top Microbiol Immunol. 2013;369:49–86. doi: 10.1007/978-3-642-27340-7_3. [DOI] [PubMed] [Google Scholar]



