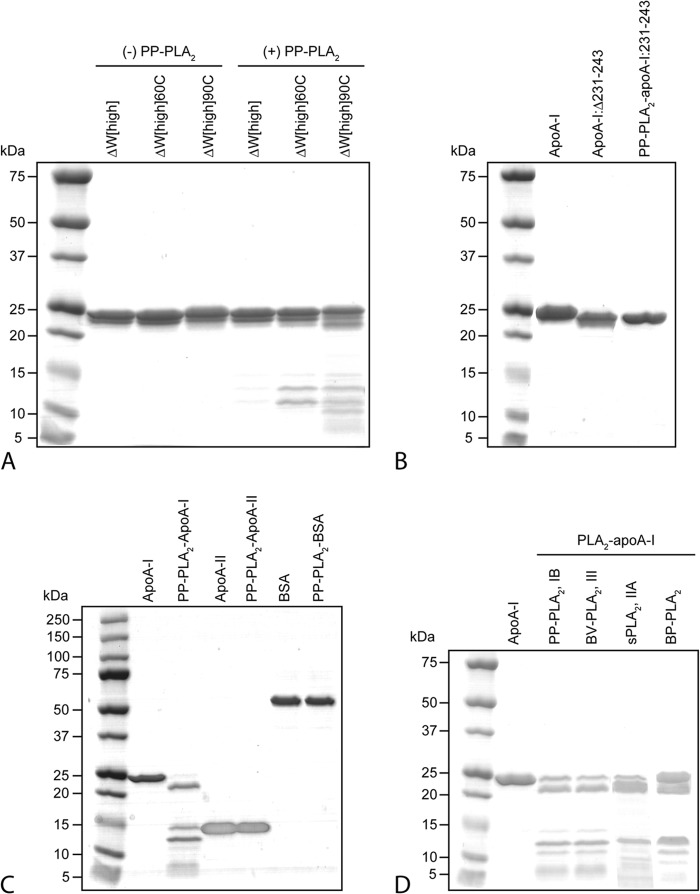
FIGURE 5.
A, characterization of lipid-free ΔW-apoA-I after incubation in the presence or in the absence of PP-PLA2. ΔW[high] is composed of high order self-association species of the protein. Thermal treatment of ΔW[high] at 60 and 90 °C produces predominantly tetramers (ΔW[high]60C) and monomers (ΔW[high]90C) of ΔW-apoA-I, respectively (40). B, effect of PP-PLA2 on the 1–230 fragment of apoA-I (apoA-I:Δ231–243) generated by a first reaction of full-length apoA-I with PLA2 and isolated by SEC. ApoA-I:Δ231–243 was incubated in the presence of PP-PLA2 for 1 h at 37 °C (PP-PLA2-apoA-I:231–243) before SDS-PAGE analysis. C, to investigate the substrate specificity of PP-PLA2 proteolytic action, lipid-free apoA-II and bovine serum albumin (BSA, essentially fatty acid-free) were incubated with PP-PLA2 in the same conditions as described for apoA-I but for long incubation times (12 h). By SDS-PAGE analysis, no fragmentation of apo-A-II or BSA was observed. D, comparison of the proteolytic activities against apoA-I of different isoforms of PLA2 and from different animal sources, by SDS-PAGE. Porcine pancreatic (PP-PLA2, type IB) and bovine pancreatic (BP-PLA2, type IB) PLA2 were incubated with apoA-I for 1 h. Bee venom (BV-PLA2, type III) and human secreted (sPLA2, type IIA) PLA2 were incubated with apoA-I for 24 h. For all panels, 5 μg of protein was loaded per lane, and molecular weight standards were Precision Plus Protein Dual Xtra standards (Bio-Rad).