FIGURE 1.
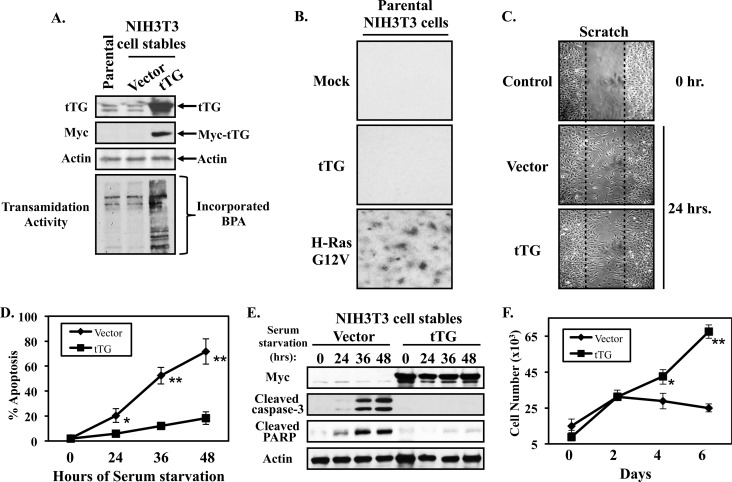
Ectopic expression of tTG in NIH3T3 fibroblasts promotes cell growth and survival. A, whole cell lysates of parental NIH3T3 cells, or NIH3T3 cells stably expressing the vector alone or a Myc-tagged form of tTG, were immunoblotted with tTG, Myc, and actin antibodies. The same cell lysates were also assayed for transamidation (cross-linking) activity by determining the incorporation of BPA into lysate proteins as described in “Experimental Procedures.” B, focus formation assays were carried-out on parental fibroblasts that were transiently transfected without (Mock), or with expression plasmids encoding either Myc-tagged tTG (tTG), or an HA-tagged activated form of Ras (H-Ras G12V). The cells were maintained in DMEM supplemented with 10% CS for 10 days, at which time they were fixed and stained with crystal violet. Shown are representative images of the resulting foci that formed for each condition. C, cell migration (scratch) assays were performed on NIH3T3 cells stably expressing the vector alone or a Myc-tagged form of tTG. Twenty-four hours after striking the wound, the cells were fixed and then visualized by light microscopy to determine the extent of wound closure. One set of vector alone-expressing fibroblasts was fixed immediately after striking the wound (Control 0 h.) to indicate the width of the initial wound (indicated by dashed lines). D, cultures of the NIH3T3 cells stably expressing the vector alone or a Myc-tagged form of tTG were placed in serum-free medium for the indicated lengths of time, at which point they were collected and stained with DAPI to identify condensed and/or blebbed nuclei. Percent apoptosis was determined by calculating the ratio of apoptotic to non-apoptotic cells. The experiments were performed in triplicate, and the results were averaged. The error bars indicate standard deviation, and the p values determined for the different conditions are as follows; *, p < 0.05 and **, p < 0.01. E, stable cell lines were cultured in serum free medium for the indicated lengths of time and lysed. The extracts were then immunoblotted with Myc, actin, cleaved caspase-3, and cleaved PARP antibodies. F, growth in low serum assays were performed on NIH3T3 cells stably expressing the vector alone or Myc-tagged tTG by plating them at a density of 2 × 104 cells/dish in 6-well dishes and then placing them in DMEM containing 0.1% CS. Every other day for 6 days, one set of cells was counted, while on the remaining sets of cells the medium was replenished. The experiments were performed in triplicate, and the results were averaged together and graphed. The error bars indicate standard deviation, and the p values determined for the different conditions are as follows; *, p < 0.05 and **, p < 0.01.