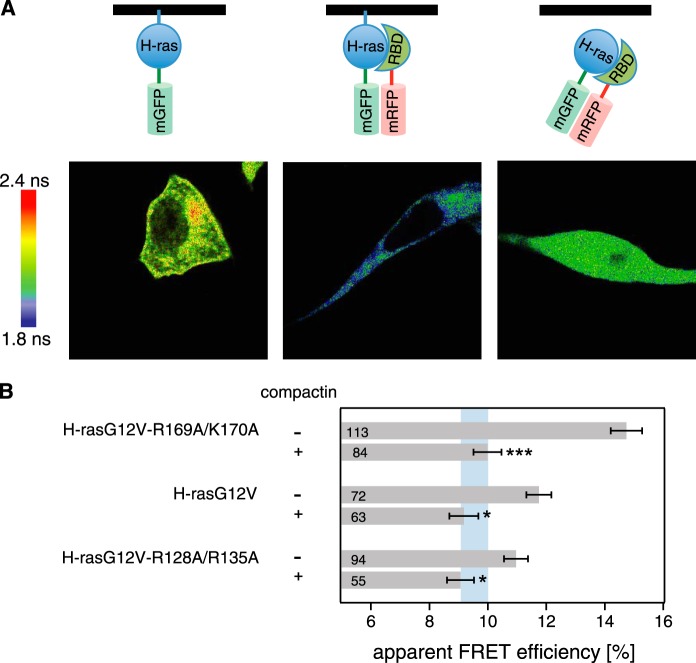
FIGURE 2.
Cytoplasmic relocalization by compactin abrogates FRET differences of H-ras orientation mutant·RBD complexes. A, confocal FLIM-FRET revealed that compactin treatment relocalized the H-rasG12V·RBD complex to the cytoplasm and led to a decrease in FRET. Imaging examples of mGFP-H-rasG12V-R169A/K170A only (left) + mRFP-RBD (center) and + mRFP-RBD and compactin treatment at a 5 μm concentration (4 h after transfection for an additional 24 h; right) are shown. Image color look-up table on the left shows fluorescence lifetimes. Schematics on the top depict the interaction situations in each experiment with the black line representing the membrane. B, interactions of pmGFP-H-rasG12V-R169A/K170A, pmGFP-H-rasG12V, or pmGFP-H-rasG12V-R128A/R135A and the acceptor mRFP-RBD in BHK cells were measured using FLIM-FRET. Treatment with 5 μm compactin was done 4 h after transfection and cells were fixed 24 h later. The apparent FRET efficiency was calculated from obtained fluorescence lifetimes. Blue vertical band represents the average ± 1 S.D. of the FRET efficiencies of conditions with compactin treatment. It would represent the baseline where there is typically no Ras signaling activity observed. Error bars correspond to the mean ± S.E., and numbers inside the bars correspond to the total number of cells imaged in each case. Three independent biological repeats were analyzed. Statistical significance was determined for the difference between compactin and non-compactin-treated samples. See under “Experimental Procedures” for details about statistical analysis (NS = nonsignificant, *, p < 0.05; **, p < 0.01; ***, p < 0.001).