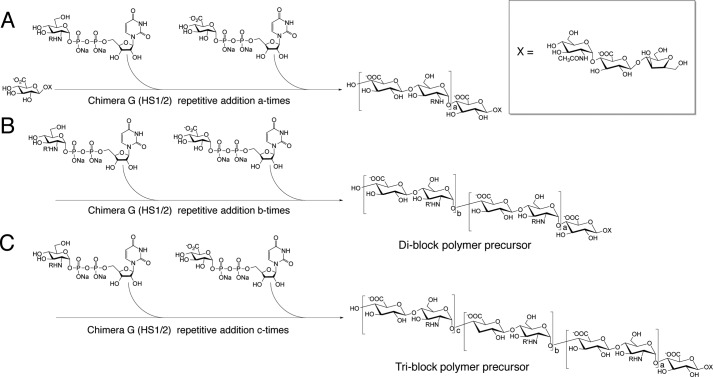
FIGURE 1.
Synthesis of di-block and tri-block HS copolymer using a chemoenzymatic approach. A, a recombinant catalyst composed of parts of both PmHS1 and -2 heparosan synthases, Chimera G, drives the elongation of a short acceptor into the first ∼8-kDa molecular mass section of the block copolymer with either protection (R = TFA) or acetylation (R = CH3CO) at the amine position of the glucosamine residue. B, after this first series of elongations, a second elongation, of the initial ∼8 kDa polymer to a total molecular mass of ∼16 kDa, is done to form a di-block copolymer. In the second series, the R′ modification of the amine residue is the reverse of the R-group in the first series. C, for tri-block copolymers, a final elongation was performed to a total molecular mass of ∼24 kDa, with R-groups identical to the first series. After the synthesis of these block copolymers, the substrates were de-NTFA-protected and N-sulfonated. Finally, the di-block and tri-block copolymers underwent treatment with C5-epimerase/2-O sulfotransferase (C5-Epi, 2-OST) and 6-O sulfotransferase isoform 1 and 3 (6-OST-1,3) in sequential steps. The sulfonated di-block and tri-block copolymers were ∼20 and 30 kDa in molecular mass, respectively (see Fig. 3B for their structures).