FIGURE 2.
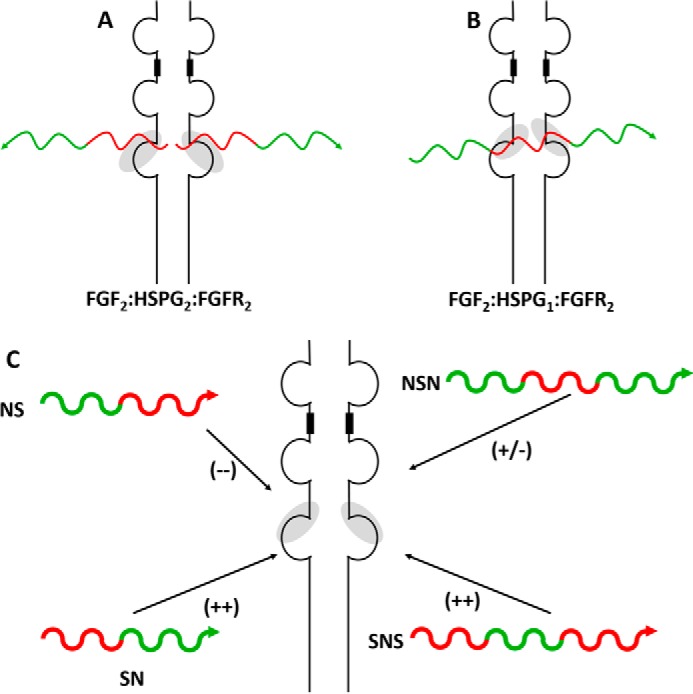
The formation of the FGF, HS, and FGFR ternary complex has previously been described by two unique mechanisms. A, the FGF2-HS2-FGFR2 model was first described by Schlessinger et al. (50) and describes a model in which the non-reducing end of two HSPGs interacts with dimeric complex of FGF2-FGFR2 to complete the ternary complex and initiate cell signaling. B, in the Pellegrini model (51), only a single molecule of HS is required for interaction with the FGF2-FGFR2 dimeric complex. In this model, the domain specificity of the sulfation pattern was less significant, as the whole HS chain was considered in the ternary complex. C, a goal of the current work is to test the domain sulfation pattern (red = high sulfation; green = low sulfation) of HS against cellular proliferation promoted by the formation of the FGF-HS-FGFR ternary complex. Each HS block copolymer is unique, in that is contains only reducing end sulfation (NS), only non-reducing end sulfation (SN), sulfation of both ends (SNS), or sulfation of neither (NSN). The arrows at the end of these substrates indicate the reducing end of the substrate. The + and − shown in the parentheses indicate the relative strength of signaling shown by each HS block copolymer.
