FIGURE 12.
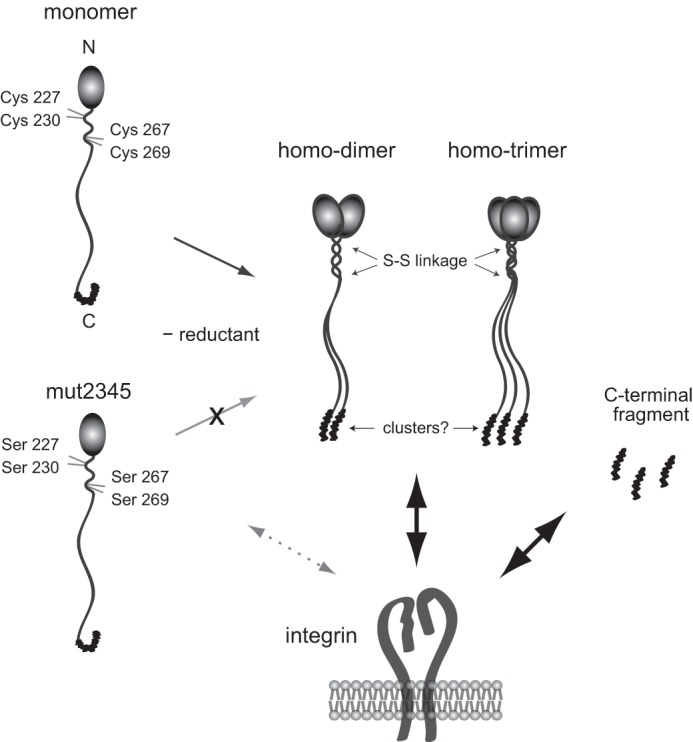
Schematic for the oligomerization and cell-binding properties of NELL1. In the absence of reductant, NELL1 forms homodimers or homotrimers through an α-helical coiled-coil formation with intermolecular disulfide bond interactions at Cys227, Cys230, Cys267, and Cys269. In contrast, the quadruple cysteine-to-serine substitution mutant exists exclusively as monomers. The C-terminal fragments and the oligomeric forms, but not monomeric forms, of NELL1 exhibit similar cell adhesion activities. An oligomerization-induced conformational change in the C-terminal region of NELL1 may be necessary for the interaction of NELL1with integrins.
