FIGURE 6.
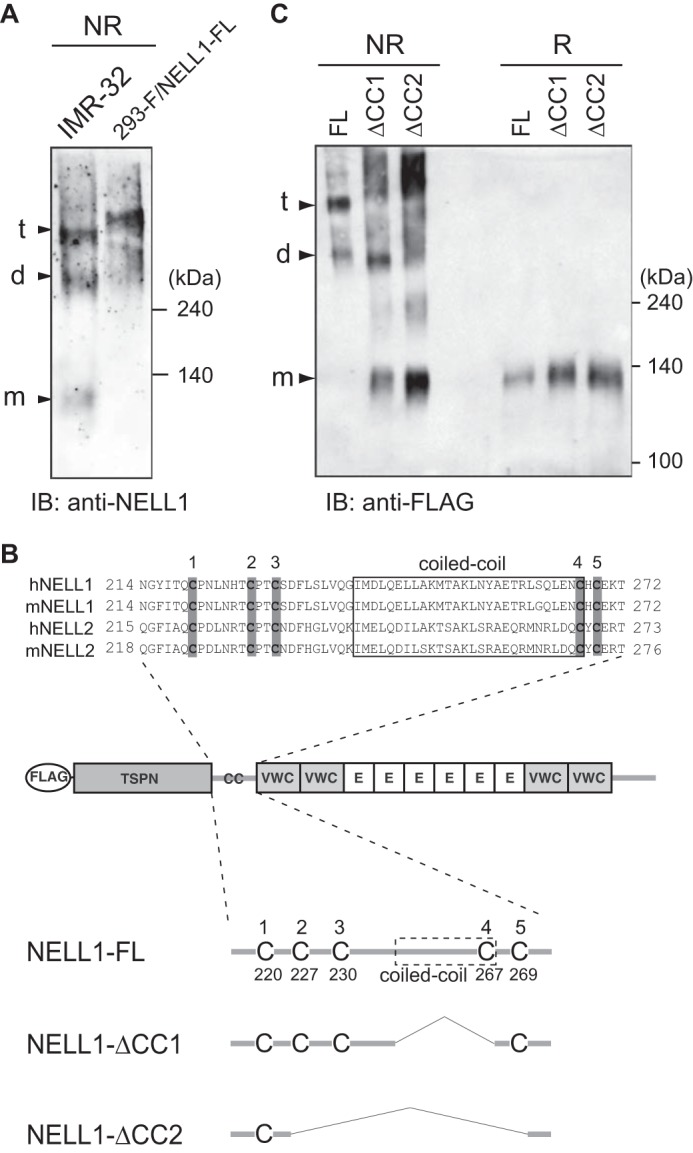
NELL1 forms homo-oligomers through the coiled-coil domain. A, oligomerization of an endogenous NELL1 protein. The conditioned media of IMR-32 cells and NELL1-FL-expressing 293-F cells were resolved by SDS-PAGE and immunoblotted (IB) with anti-NELL1 antibody under non-reducing (NR) conditions. The bands assumed to be monomers (m), dimers (d), and trimers (t) are indicated at the left. Molecular weight markers are indicated at the right. B, schematic of full-length NELL1 protein and its deletion mutants lacking the coiled-coil domain. A FLAG tag was fused to the N terminus of NELL1. The amino acid sequences around the coiled-coil domains of human and mouse NELL proteins were aligned at the top. The coiled-coil domains, which were identified using the SMART program (Simple Molecular Architecture Research Tool) (44, 45), are boxed. The five conserved cysteine residues are shaded in gray. E, EGF-like repeat. C, oligomerization of NELL1-FL and its deletion mutants. NELL1-FL and its deletion mutants were expressed in 293-F cells. The conditioned media were resolved by SDS-PAGE and immunoblotted with anti-FLAG antibody under non-reducing or reducing (R) conditions.
