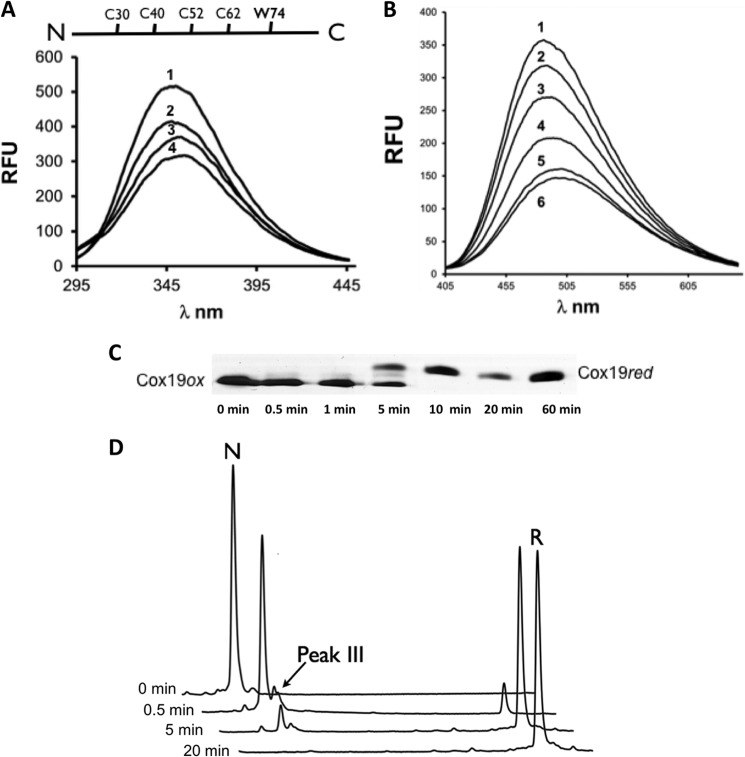
FIGURE 1.
Reductive unfolding of Cox19 involves single disulfide intermediates. A, Cox19 reduction and unfolding can be monitored by Cox19 W74 fluorescence. RFU, relative fluorescence units. DTT 20 mm was added to oxidized Cox19 and W74 emission spectrum recorded at time 0 (1), 30s (2), 2min (3), 10 min (4). B, Cox19 reduction and unfolding can be monitored by ANS fluorescence. DTT 20 mm was added to oxidized Cox19 in the presence of ANS (100 mm), and the probe emission spectra was recorded at time 0 (1), 30s (2), 1 min (3), 2 min (4), 10 min (5), and 25 min (6). C, reduction of fully oxidized Cox19 followed using AMS thiol mobility assay. In addition to the bands of the fully oxidized and fully reduced protein, an intermediate band was observed corresponding to a single disulfide intermediate. D, species involved in Cox19 reductive unfolding can be separated by RP-HPLC following reaction quenching with TFA, which blocks subsequent oxidation and reshuffling. Oxidized Cox19 (N), because of its compact structure compared with the reduced protein (R), has a shorter elution time. A small peak eluting close to N was identified at intermediate time points, probably corresponding to a single disulfide species (see below).