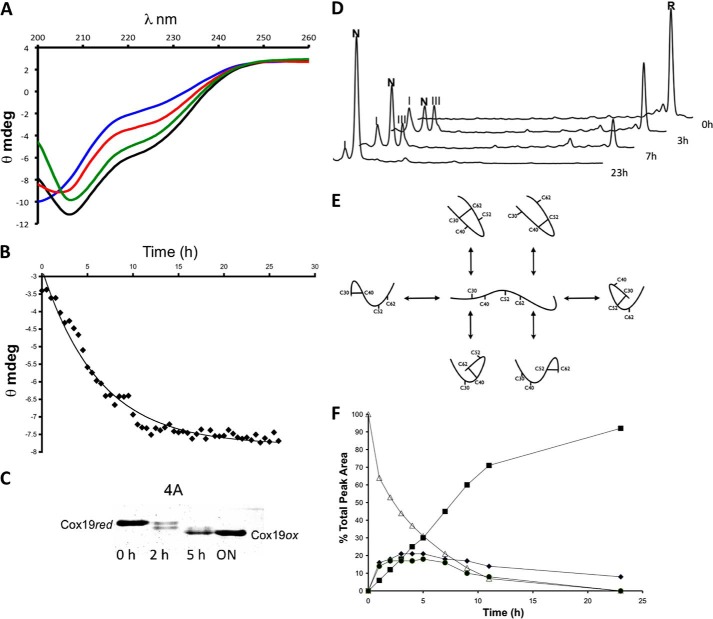
FIGURE 2.
Slow oxidation of Cox19 results in folding. A, Cox19 was reduced in 20 mm DTT for at least 3 h. The reduced protein was loaded in a small PD10 column containing a G25 resin. The recovered protein, free of DTT, was allowed to air oxidized at 25 °C, and CD spectra were taken at 1-h intervals. Reduced Cox19 (blue, time 0) display features of an unfolded protein but slowly recovers features characteristic of a folded protein. Shown are the spectra at 4 (red), 8 (green), and 26 h (black). B, ellipticity at 215 nm, was plotted as a function of time. C, Cox19 air oxidation was followed using the AMS thiol shift assay. Full Cox19 oxidation takes about 20 h to occur, and the presence of single disulfide intermediates is clearly visible. D, time-dependent Cox19 oxidation was followed by RP-HPLC. Cox19 oxidation (R, fully reduced protein) displays slow kinetics and involves the formation of at least two intermediates eluting close to the native protein. Those peaks are identified as I (Cys40-Cys62) and III (Cys40-Cys52), N represents native protein. E, Cox19 contains 4 Cys residues arranged in two CX9C motifs that can form a maximum number of 6 different single disulfide species; only the Cys30-Cys62 and Cys40-Cys52 intermediates contain native disulfide bonds. F, peaks corresponding to Cox19 folding species were integrated, and their area plotted as a function of time, reduced Cox19 (▵), Native Cox19 (■), Peak I (♦), and Peak III (●). Peaks I and III display kinetics characteristics of folding intermediates as their formation precedes native protein accumulation.