Figure 2.
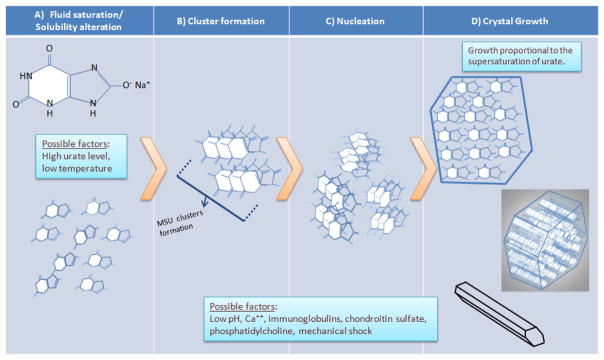
Hypothetical model of the initiation and propagation of monosodium urate crystallization. A) Monosodium urate molecules remain fully in solution until an event that changes their solubility (e.g., increased concentration and decreased temperature, as discussed in the text). B) Soluble monosodium urate molecules begin to cluster while still in solution. C) Clusters aggregate into crystal nuclei, the basis for additional crystal formation and growth. D) Formation of fully-aggregated monosodium urate crystals. Top, monosodium urate in the crystals are arranged in flat sheets as seen in cross-section. Middle, the surface of these flat sheets forms the major growth faces of the crystal, with the flat sheets stacking along the crystal’s long axis. Bottom, fully formed (but potentially still growing) crystal, whose three axes are non-perpendicular (i.e., triclinic structure). Local factors may promote nucleation and/or crystal growth (e.g., pH and others; see text)
