Fig. 1.
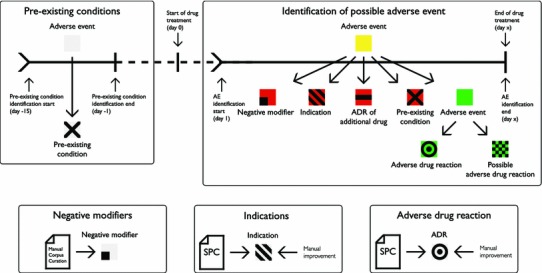
AE extraction and data integration. AEs were extracted between drug introduction and discontinuation, where we filtered out AEs if the text in the clinical note suggests it did not happen, affected someone else or happened in the past. Additionally, we filtered all indications of the drug and ADRs related to additional drugs. Finally, all pre-existing conditions were removed. Remaining AEs were sorted into ADRs and possible ADRs; the latter was presented for manual review. ADR adverse drug reaction, AE adverse event, EPR electronic patient record, SPC Summary of Product Characteristics
