Abstract
Background
Kaposi sarcoma–associated herpesvirus (KSHV) infection is endemic among adult populations in Africa. A prevailing view is that childhood transmission is primarily responsible for the high seroprevalence of KSHV among adults that is observed throughout the continent. However, few studies have directly examined children, particularly in locations where KS is not commonly endemic.
Methods
Participants were children aged 1.5−8.9 years, including 427 children from a population-based sample in South Africa, 422 from a population-based sample in Uganda, and 567 from a clinic-based sample in Uganda. All serum specimens were tested by the same laboratory for KSHV antibodies with use of 2 enzyme immunoassays (against K8.1 and ORF65) and 1 immunofluorescence assay.
Results
KSHV seroprevalence was 7.5%−9.0% among South African children and was not associated with age. In contrast, in the Ugandan population-based sample, KSHV seroprevalence increased from 10% among 2-year-old children to 30.6% among 8-year-old children (Ptrend < .001). In the Ugandan clinic-based sample, seroprevalence increased from 9.3% among 2-year-old children to 36.4% among 8-year-old children (Ptrend < .001).
Conclusion
Two distinct relationships between age and KSHV infection among children imply that KSHV transmission among children is not uniform throughout Africa and is therefore not always responsible for the high seroprevalence observed in adults. There are at least 2 patterns of KSHV transmission in Africa.
Since its identification in 1994 [1], Kaposi sarcoma–associated herpesvirus (KSHV), or human herpesvirus 8, has been established as the etiologic agent of KS [2–6]. Consistent with its etiologic role, KSHV seroprevalence has generally, where studied, paralleled that of KS incidence. Specifically, in the United States, the prevalence of KSHV is low in the general population (<5%) but not among homosexual men, among whom the occurrence of KS and KSHV is much more common [2, 3, 7–11]. In contrast, KSHV infection is common in the Mediterranean, the Middle East, and Africa—all areas where KS occurs more frequently in the general population, even without concurrent human immunodeficiency virus (HIV) infection. Indeed, KSHV seroprevalence is highest in sub-Saharan Africa, where estimates among adults range from 20% to 70% [12–23].
Although it has been established that KSHV infection is endemic among most sub-Saharan African adult populations, how and when KSHV is transmitted within Africa and whether seroprevalence tracks KS incidence is poorly understood. A prevailing view is that horizontal transmission throughout childhood is an important means of KSHV transmission and that this occurs uniformly in the continent, irrespective of underlying KS incidence [24–30]. However, few studies have directly examined children, particularly in population-based samples that compare areas where the endemic form (non–HIV related) of KS is uncommon versus where it is common. Further, although many KSHV seroprevalence studies have been performed in discrete areas throughout sub-Saharan Africa [12–19], comparing seroprevalence between regions from different studies is problematic because of the diverse serologic assays used and documented deficiencies in interassay agreement [31].
To directly characterize the epidemiology of KSHV among children across different African regions, we compared KSHV seroprevalence among children in a region where endemic KS is uncommon (South Africa) to that in a region where endemic KS is common (Uganda) [32]. To enhance the validity of the comparison, we evaluated population-based samples in both regions with KSHV antibody testing standardly performed in the same laboratory. In addition, our study provided the opportunity to examine the role of other correlates of KSHV infection in children and age-related patterns in KSHV antibody reactivity.
METHODS
Study Population
Participants were sampled from 4 sources. The first 2 were in South Africa where, in 2003, 2 communities were studied. These were Cato Manor, an urban settlement in Durban, and KwaXimba, a rural area 45 km outside of Durban. In both communities, we sampled children aged 2, 4, and 8 years and their primary female caregiver. Because records of all households were not available, it was not possible to use conventional probability sampling. Instead, households were randomly selected using the following approach. First, we examined aerial photographs and, with use of roads as landmarks, circumscribed the 2 communities and divided each into sections. Households from each section were sampled by 5 teams, each of which carried a bag of chips numbered 1 to 100. Each team started from a randomly selected viewpoint (eg, a point on a hilltop) and pulled a chip for every household that they could see from the viewpoint. If the chip was numbered 1−20, the household was approached. At each household, the team determined whether a child aged 2, 4, or 8 years resided within. If there was an eligible child, she/he and her/his primary female caregiver were recruited. In households with >1 eligible child, 1 child was randomly selected. Of children deemed eligible, 89% in Cato Manor and 95% in KwaXimba were enrolled.
The third source of participants was a population-based cohort originally assembled for malaria investigation in Kampala, Uganda [33]. In brief, a probability sample was obtained by Global Positioning System–assisted enumeration of each household in the Mulago III Parish in 2004. A random list of households with at least 1 child aged <10 years was generated, and all children aged <10 years in each selected household were eligible. Children with a known chronic disease or severe malnutrition were excluded. Of eligible children, 81% enrolled. For the present study of KSHV, children aged <1.5 or >9 years were excluded.
The fourth source of participants was a clinic-based study of KSHV transmission by blood transfusion in Kampala in 2001 [34]. This source was included to provide an opportunity for direct comparison in the same region with a population-based sample (from Mulago III Parish). In brief, consecutive children who were admitted to Mulago Hospital, who had no history of blood transfusion within the prior 6 months, and who received a blood transfusion were enrolled. For the present analysis, children aged <1.5 or >9 years were excluded, and the blood specimen obtained before transfusion was evaluated. In each study, informed consent was obtained from the child's caregiver.
Measurements
Demographic characteristics
Age and sex data were collected by interviewer-administered questionnaire.
KSHV antibody testing
For all participants, blood samples were tested for antibodies to KSHV with 2 enzyme immunoassays (EIAs) and 1 indirect immunofluorescence assay (IFA); each was previously described and performed by the same personnel at the Centers for Disease Control and Prevention (CDC). The 2 EIAs (EIA-K8.1-synthetic and EIA-ORF65-synthetic) target antibodies to open reading frame (ORF) K8.1 and ORF 65, respectively, with use of synthetic peptides as antigen substrates [35]. The IFA uses KSHV-infected BCBL-1 cells as an antigen substrate, in which KSHV is induced to its replicative phase [36]; specimens were evaluated at a dilution of 1:80. Specimens that were reactive in any 2 tests or were positive in the IFA alone were classified as KSHV antibody positive. Specimens that were nonreactive in all tests or reactive in only 1 EIA were categorized as KSHV antibody negative; all other patterns were considered equivocal and were excluded from analysis. This serologic algorithm has an estimated specificity of 97.5%, as evidenced by testing blood donors [37], and a sensitivity of 96.3%, as evidenced by testing patients with KS [38]. For purposes of independent laboratory confirmation, specimens from South Africa were tested at the National Cancer Institute with use of 2 EIAs with recombinant antigens (EIA-K8.1-recombinant and EIA-ORF73-recombinant) described elsewhere [22, 39]. Specimens with reactivity in either assay were deemed to be overall KSHV antibody positive; there were no equivocal classifications.
Other antibody testing
Among a random sample of South African children, antibodies to Epstein-Barr virus (EBV) were assessed using a viral capsid antigen EIA (DiaSorin), antibodies to cytomegalovirus (CMV) were assessed using a whole virus EIA (Quest International), and antibodies to hepatitis A virus (HAV) were assessed using Abbott HAVAB EIA (Abbott Laboratories).
Statistical Analysis
Our primary analysis compared KSHV seroprevalence—as derived from testing at the CDC with the IFA, EIA-K8.1-synthetic, and EIA-ORF65-synthetic—among children in South Africa and Uganda. Logistic regression was performed to adjust for confounding and to assess for potential effect modification by age, sex, and HIV infection status. Because some children in the Ugandan population-based sample lived in the same household, it could not be assumed that their KSHV infection status was independent. Therefore, when analyzing this sample, we used the generalized estimating equations approach [40]. To confirm inferences generated using the IFA, EIA-K8.1-synthetic, and EIA-ORF65-synthetic, a secondary analysis evaluated KSHV antibody testing performed on specimens from South African children with use of the EIA-K8.1-recombinant and EIA-ORF73-recombinant at the National Cancer Institute. Analyses were conducted using Stata, version 9.1 (StataCorp), and SAS, version 9.1 (SAS Institute).
RESULTS
Specimens were available from 1470 children, including 461 in South Africa and 1009 in Uganda. Overall, 54 (3.7%) were classified as equivocal by KSHV antibody testing and were not considered for further analysis. Of the 1416 children with unequivocal results, 427 (201 urban and 226 rural) were from South Africa (median age, 4.4 years; interquartile range [IQR], 2.6−8.1 years), 422 were from the Ugandan population-based study (median age, 5.6 years; IQR, 3.9−7.5 years), and 567 were from the Ugandan clinic-based study (median age, 2.5 years; IQR, 1.9−4.3 years) (table 1). Approximately one-half of the children from both countries were female. In both countries, 6%−7% of participants were HIV infected, but this varied within Uganda (0.3% in the population-based sample and 10.8% in the clinic sample).
Table 1.
Characteristics of study participants.
| South Africa |
Uganda |
|||||
|---|---|---|---|---|---|---|
| Characteristic | Population based (urban) (n = 201) | Population based (rural) (n = 226) | All (n = 427) | Population based (n = 422) | Clinic based (n = 567) | All (n = 989) |
| Median age (IQR), years | 4.4 (2.6−7.9) | 4.4 (2.6−8.2) | 4.4 (2.6−8.1) | 5.6 (3.9−7.5) | 2.5 (1.9−4.3) | 3.7 (2.2−6.0) |
| Female sex | 53.2 | 54.1 | 53.8 | 54.5 | 49.0 | 51.4 |
| HIV infected | 7.0 | 5.8 | 6.4 | 0.32 | 10.8 | 7.0 |
NOTE. Data are percentage of patients, unless otherwise indicated. IQR, interquartile range.
Effect of country and age on KSHV seroprevalence
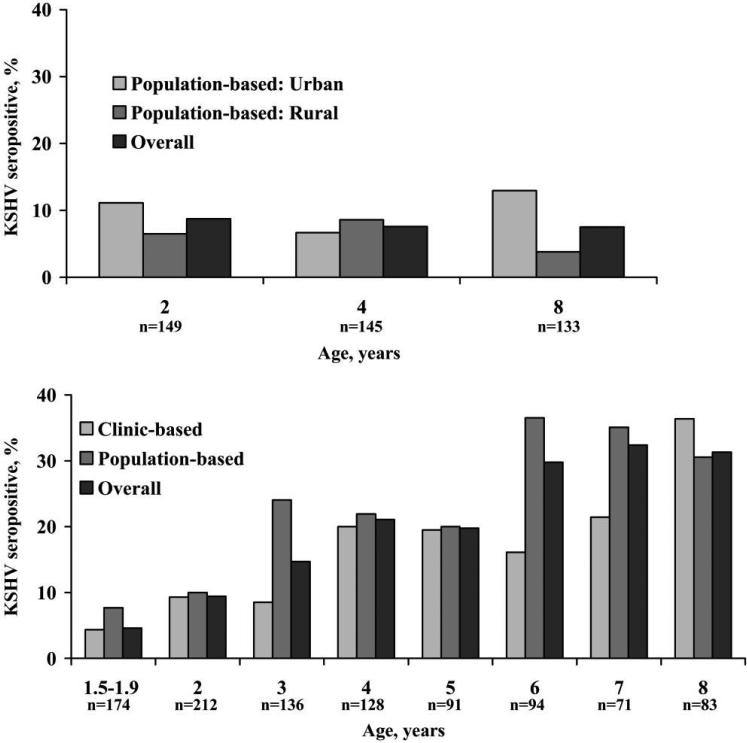
In South Africa, KSHV seroprevalence was 7.5%−9.0% among children of all ages (pooling across urban and rural settings), and there was no statistical evidence for a change in seroprevalence with age (Ptrend = .80) (figure 1). In contrast, in the Ugandan population-based study, KSHV seroprevalence was 10.0% among 2-year-old children and increased to 30.6% among 8-year-old children (Ptrend < .001). In the Ugandan clinic-based sample, KSHV seroprevalence was 9.3% among 2-year-old children and increased to 36.4% among 8-year-old children (Ptrend < .001). Adjustment for HIV infection status and sex did not change the relationship between age and KSHV seroprevalence in South Africa (Ptrend = .62) or Uganda (Ptrend ≤ .001, for both samples). The difference in effect of age on KSHV prevalence between South Africa and Uganda was reflected in the P value for interaction (P < .001) (table 2). Altering the interpretation of the serologic algorithm did not change the results. Assigning an overall positive result to any specimen that was reactive in at least 1 assay resulted in KSHV seroprevalence of 12.9%, 11.0%, and 9.0% in South African children and 10.2%, 20.6%, and 31.7% among Ugandan children aged 2, 4, and 8 years, respectively.
Figure 1.
Kaposi sarcoma–associated herpesvirus (KSHV) seroprevalence among children in South Africa (top) and Uganda (bottom), by age. n values underneath bars refer to the overall number of children in each age group.
Table 2.
Determinants of Kaposi sarcoma–associated herpesvirus infection in children in South Africa and Uganda.
| South Africa |
Uganda |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI)a | P | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI)a | P | P for interactionb |
| Age, per 1 year increase | 0.98 (0.85−1.1) | .80 | 0.96 (0.81−1.1) | .64 | 1.3 (1.2−1.4) | <.001 | 1.3 (1.2−1.4) | <.001 | <.001 |
| Sex | |||||||||
| Female | Reference | Reference | Reference | Reference | |||||
| Male | 0.45 (0.18−1.1) | .09 | 0.44 (0.18−1.1) | .08 | 0.90 (0.58−1.4) | .62 | 0.96 (0.66−1.4) | .82 | .14 |
| HIV infection status | |||||||||
| Uninfected | Reference | Reference | Reference | Reference | |||||
| Infected | 2.2 (0.72−6.8) | .17 | 0.93 (0.12−7.5) | .95 | 0.17 (0.04−0.70) | .01 | 0.22 (0.06−0.87) | .03 | .22 |
| Study Site | |||||||||
| South Africa | |||||||||
| Rural | Reference | Reference | ... | ... | |||||
| Urban | 1.7 (0.82−3.4) | .16 | 1.5 (0.63−3.5) | .36 | ... | ... | |||
| Uganda | |||||||||
| Clinic based | ... | ... | Reference | Reference | |||||
| Population based | ... | ... | 2.7 (1.9−3.9) | <.001 | 1.5 (0.94−2.4) | .09 | |||
NOTE. CI, confidence interval; OR, odds ratio.
Adjusted for all characteristics.
The P for interaction term indicates whether the magnitude of association between a specific characteristic and Kaposi sarcoma–associated herpesvirus seropositivity, after adjusting for all other characteristics, differed between the Ugandan and South African populations. A small P value is indicative of a difference between populations, and a large Pvalue is indicative of no evidence for a difference.
Additional evaluation of South African children
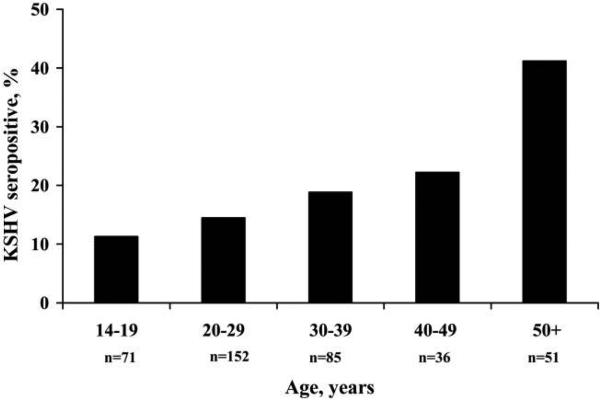
Because of the notable difference in KSHV seroprevalence by age between South African and Ugandan children, we further evaluated the South African specimens with KSHV antibody testing at an independent laboratory, to confirm physical integrity and to detect any unusual sample characteristics. Use of EIA-K8.1-recombinant and EIA-ORF73-recombinant, which were performed at the National Cancer Institute, did not alter the results: KSHV seroprevalence was again <9% in all age groups (8.1%, 8.3%, and 7.5% among children aged 2, 4, and 8 years, respectively), and there was no evidence for a change in seroprevalence with age (Ptrend = .81). To assess physical specimen integrity, we tested for antibodies against EBV and CMV—2 pathogens for which we expected a high seroprevalence. Among a random sample of 90 South African children, 95.6% were EBV antibody positive and 94.4% were CMV antibody positive, indicating satisfactory specimen integrity. There were no significant differences in seroprevalence of EBV (Ptrend = .83) or CMV (Ptrend = .13) by age. As an indication of the representativeness of the sample of children from South Africa, we evaluated the age dependence of HAV seropositivity. We tested for antibodies against HAV among 150 randomly selected children aged 2 and 8 years; 23 (32.4%) of 71 children aged 2 years and 73 (92.4%) of 79 children aged 8 years were HAV antibody positive (P < .001). As further evidence of the representativeness of the sampling, we observed a clear age-dependent increase in KSHV seroprevalence among the adult caregivers of the children. Of the caregivers of children included in the present analysis (n = 427), KSHV antibody results were available for 424 caregivers, 395 of which had nonequivocal results. KSHV seroprevalence was 11.3% among adults aged <20 years and increased to >40.0% among adults aged ≥50 years (Ptrend < .001) (figure 2).
Figure 2.
Kaposi sarcoma–associated herpesvirus (KSHV) seroprevalence among adult female caregivers in South Africa. n values underneath bars refer to the overall number of persons in each age group.
Other determinants of KSHV serostatus in children
In addition to the role of age and country of residence, we evaluated the independent role of other potential determinants of KSHV infection in children, including sex, HIV infection status, and study setting (table 2). In neither country was there strong evidence for an association of KSHV with sex. In South Africa, there was no strong evidence for an association between HIV infection status and KSHV seropositivity (odds ratio [OR], 0.93; 95% confidence interval [CI], 0.12−7.5; P = .95), but in Uganda, HIV-infected children were significantly less likely to be KSHV infected (OR, 0.22; 95% CI, 0.06−0.87; P = .03). Of note, the association between HIV infection status and KSHV infection in Uganda was entirely driven by the children in the clinic-based sample (OR, 0.20; 95% CI, 0.05−0.88; P = .03), because there were too few HIV-infected children in the population-based sample (n = 1) to be influential. Finally, in South Africa, there was no significant difference in KSHV seroprevalence between urban and rural venues (P = .36). In Uganda, children in the clinic-based sample were not more likely to be KSHV-infected than those in the population-based sample (P = .09).
Patterns of reactivity in KSHV antibody assays according to age
Among participants deemed KSHV antibody positive by our primary serologic algorithm (with the IFA, EIA-K8.1-synthetic, and EIA-ORF65-synthetic performed at the CDC), we assessed age-related patterns of reactivity in the 3 assays that are included in the algorithm. Of the 170 Ugandan children aged 1.5−9 years who were deemed overall KSHV antibody positive, we observed a decreasing isolated reactivity in the IFA with increasing age. A total of 16 (57.1%) of 28 children aged 1.5−2 years, 22 (33.9%) of 65 children aged 3−5 years, and 18 (23.4%) of 77 children aged 6−8 years demonstrated reactivity in the IFA alone (Ptrend = .002) (table 3). Accordingly, with increasing age, we observed a broadening of assay reactivity, with 3 (10.7%) of 28 children aged 1.5−2 years, 8 (12.3%) of 65 children aged 3−5 years, and 16 (20.8%) of 77 children aged 6−8 years reactive to all 3 assays used (Ptrend = .12). A similar pattern was observed among the 109 South African children and adults who were found to be KSHV antibody positive (34 children and 75 adults), but the contrast was only apparent when comparing all children to the adults (table 3). In South Africa, 26 (76.5%) of 34 children and 23 (30.7%) of 75 adults demonstrated isolated reactivity in the IFA (P < .001), whereas 3 (8.8%) of 34 children and 30 (40.0%) of 75 adults were reactive in all 3 assays (P < .001).
Table 3.
Patterns of reactivity in Kaposi sarcoma–associated herpesvirus (KSHV) antibody assays among KSHV antibody–positive participants, by age.
| Reactivity pattern | Uganda, no. (%) of participants | South Africa, no. (%) of participants | |||||||
|---|---|---|---|---|---|---|---|---|---|
| EIA-ORF 65 | EIA-ORF K8.1 | IFA | 1.5−2 years (n = 28) | 3−5 years (n = 65) | 6−8 years (n = 77) | 2 years (n = 13) | 4 years (n = 11) | 8 years (n = 10) | Adult (n = 75) |
| − | − | + | 16 (57.1) | 22 (33.9) | 18 (23.4) | 11 (84.6) | 7 (63.6) | 8 (80.0) | 23 (30.7) |
| − | I | + | ... | 1 (1.5) | ... | ... | ... | ... | ... |
| − | + | + | 8 (28.6) | 31 (47.7) | 36 (46.8) | 1 (7.7) | 2 (18.2) | 1 (10.0) | 20 (26.7) |
| I | − | + | ... | ... | 1 (1.3) | ... | ... | ... | ... |
| I | + | + | ... | 1 (1.5) | 1 (1.3) | ... | ... | ... | ... |
| + | + | − | ... | ... | ... | ... | ... | ... | 1 (1.3) |
| + | − | + | 1 (3.6) | 1 (1.5) | 5 (6.5) | 1 (7.7) | ... | ... | 1 (1.3) |
| + | + | I | ... | 1 (1.5) | ... | ... | ... | ... | ... |
| + | + | + | 3 (10.7) | 8 (12.3) | 16 (20.8) | ... | 2 (18.2) | 1 (10.0) | 30 (40.0) |
NOTE. +, reactive; −, nonreactive; EIA, enzyme immunoassay performed with synthetic peptides; I, indeterminate result; IFA, immunofluorescence assay. All assays were performed at the Centers for Disease Control and Prevention.
DISCUSSION
We directly compared KSHV seroprevalence in a population-based sample of children in Uganda, where endemic KS is common, to children in South Africa, where endemic KS is uncommon [32]. To mitigate the effects of interassay variability, which has been an obstacle to comparing prior studies of KSHV seroprevalence, we used the same serologic assays performed in the same laboratory in both populations. With these population-based samples and standardized approach to antibody testing, we found 2 distinct relationships between age and KSHV seropositivity. In South Africa, KSHV seroprevalence was relatively low among children and did not increase with age. In contrast, in Uganda, KSHV seroprevalence increased to >30% among children aged 8 years. These 2 distinct relationships imply that KSHV transmission is not monolithic among African children, and that there are at least 2 epidemiologic patterns of transmission in the continent.
Our lack of association between age and KSHV seropositivity among South African children is seemingly in contrast to our previous results [21, 23]. Indeed, we performed additional testing because our findings were different. First, we ruled out a loss of specimen physical integrity, because seroprevalence for CMV and EBV was as expected. Second, we ruled out the possibility that our specimens from South Africa had, by chance, been obtained from a nonrepresentative population, because HAV seroprevalence was as expected for Africa. Third, KSHV antibody testing at an independent laboratory yielded the same result.
Closer evaluation of prior work may help to explain the differences. One of the first reports of age dependence was observed in a relatively small sample (N = 111) of South African children involved with paternity testing, among whom the relationship between age and KSHV infection was driven by inclusion of children aged 10−14 years [21]. Among children of this age, sexual transmission cannot be excluded, and when an analysis is limited to younger children, there is not a significant association. Furthermore, in a recent larger sample of this group of children (N = 1287), no relationship was observed between age and KSHV infection [22]. In another study, in which antibody testing was performed on blood spots, it is notable that the age relationship depended on the assay used for assessment [23]. Among the predominant segment of the study population—the HIV-uninfected children (94% of the sample, which matched our percentage of HIV-uninfected children in South Africa)—there was a relationship between age and KSHV seropositivity when using an EIA for ORF K8.1, but there was not a substantive relationship when using an IFA against latency-associated nuclear antigen [23]. We speculate that this discrepancy may be explained by a lack of specificity in the EIA when using blood spots, a fact supported by the authors’ report that the specificity of the EIA was 70% and that of the IFA was 90% when compared to a matched serum sample. Thus, when limiting inferences to those obtained from the latency-associated nuclear antigen IFA among HIV-uninfected children, the KSHV seroprevalence in this study (8%−9% among children aged 1 to >6 years) is remarkably similar to that in our study and is notably not related to age. Overall, our observations and the revisited work of others do not support ongoing KSHV transmission throughout childhood in South Africa and do suggest that most transmission occurs after puberty.
Although we believe that the lack of association between age and KSHV seroprevalence in South Africa argues against ongoing KSHV transmission in childhood, a question remains as to what explains the unwavering 7%−9% seroprevalence that we observed. There are at least 5 possible explanations. First, it is conceivable that the persistent seroprevalence is attributable to vertical infection, but this is unlikely because vertical KSHV infection is rare [41]. Second, it is possible that horizontal transmission occurred at an early age (<2 years) but that only a small genetically susceptible fraction became infected. This scenario is unlikely, because the adult caregivers of these children exhibit much higher seroprevalence and age-dependent KSHV seroprevalence, indicating their robust susceptibility. Third, it is possible that ongoing incident infection throughout childhood is being negated by seroreversion. Although KSHV seroreversion has been documented [42], it is very unlikely to be occurring at the same rate as new infection. Fourth, a flat pattern of KSHV seroprevalence could occur if KSHV was just introduced. However, KSHV has likely been in sub-Saharan Africa for >100,000 years [43]. Fifth, and what we believe is most likely, is that possibly one-third to one-half of the observed seroreactivity is age-independent nonspecific reactivity (ie, false positives) that obscures the underlying seroprevalence, which is likely very low.
In contrast to our observations in South Africa, we observed a monotonic increase in KSHV seropositivity with age among Ugandan children. This age-dependent pattern, observed in both the population-based and clinic-based samples, is consistent with results of other studies [19, 27–30]. This pattern suggests that the primary mode of KSHV transmission in this region is horizontal and nonsexual during childhood and that there is ongoing transmission throughout childhood.
Although our primary focus was on age and regional differences in KSHV seroprevalence, we also evaluated the role of several other factors. In terms of sex, we did not observe significant differences in KSHV seroprevalence, consistent with results reported elsewhere [20, 22, 28, 44]. Regarding HIV infection status, we lacked sufficient power in South Africa to detect anything but large differences. In Uganda, however, we found that HIV apparently protected against KSHV infection. To our knowledge, whereas prior work has shown a positive association between KSHV and HIV infection [22–24, 45] or no association [26, 46], none has demonstrated a protective role of HIV infection. We do not believe that this represents biologic protection conferred by HIV but rather that the finding is attributable to chance or perhaps the behavior of children with HIV infection, who may have reduced close contact with others (eg, their mothers may have died of HIV infection). Finally, within each region, we had the opportunity to perform a unique analysis. In South Africa, our design allowed for a comparison between urban and rural settings, but we found no differences. In Uganda, contrary to what we expected, KSHV seroprevalence was not significantly higher in the clinic sample.
The large number of KSHV-seropositive children in Uganda also allowed us to evaluate age-related patterns of reactivity in different KSHV antibody assays. We observed decreasing isolated reactivity of the IFA and a broadening of reactivity to all 3 assays with increased age. One interpretation, supported by results reported elsewhere [42], is that the IFA is more sensitive to the earliest stages of infection, probably because of the wider array of antigens it presents. If true, this has important implications for investigation of seroconversion that seeks to accurately determine the time of KSHV acquisition.
The primary strengths of our work are the population-based nature of our samples and our standardized approach to antibody testing. To our knowledge, this is among the first studies to compare population-based samples across African geographic regions with use of the same serologic algorithm. The primary limitation is that we were restricted to 2 discrete regions. However, we do not believe that the lack of association between age and KSHV seropositivity in South Africa is an anomaly, because we observed the same pattern in geographically separated rural and urban communities. Moreover, a recent report that sampled all 9 provinces of South Africa, had similar findings [22].
Although we conclude that at least 2 epidemiologic patterns of KSHV transmission exist among children in Africa, the question looms as to why 2 regions that are not that far apart geographically would have such disparate patterns. Potential explanations include differences in host genetic susceptibility [47, 48], KSHV genotypic differences [43], environmental cofactors [13, 49, 50], or even theoretical differences in behaviors that may affect KSHV transmission. Determining the explanation may provide a clue about how to prevent KSHV transmission. Finally, we note that the 2 patterns of KSHV transmission that we have observed track with the underlying incidence of endemic KS in their respective populations. Whether KSHV infection in childhood is a critical determinant for KS in adulthood and is in part responsible for the high incidence of endemic KS observed in areas such as Uganda—similar to hepatitis B virus infection in childhood and hepatocellular carcinoma as an adult—merits investigation.
Acknowledgments
We are grateful to Minal Amin (CDC) for excellent technical assistance and the communities of KwaXimba and Cato Manor in South Africa and Mulago III Parish for welcoming our study teams. We thank the following field and laboratory staff for their efforts. South Africa: S. Maphumulo, T.I. Mbumbe, B. Mkhize, M.I. Mlaba, M.I. Mngadi, D. Ndlovu, E.S.B. Ndlovu, J.R. Ngcobo, S. Nzuza, M. Peta, and N.N. Pheta; HIV Pathogenesis Programme, University of KwaZulu Natal: D. Ramduth, N. Ntuba, N. Nene, N. Mngquandaniso, and I. Honeyborne. Uganda Mulago III Parish Study: C. Akello, N. Asaba, S. Balikowa, A. Balita, S. Bakeera-Kitaka, C. Bako, K. Banek, J. Bbosa, A. Birabwa, C. Bongole, T. Clark, M. Dongo, C. Egibwa, F. Jurua, R. Kabuleta, K. Kahiigwa, I. Kalyesubula, B.M. Karakire, M. Kasanga, F. Kateera, M. Kiggundu, N. Kilama, F. Kintu, S. Kibirango, G. Kizito, C. Maiteki, K. Maxwell, N. Mbabazi, R. Mbabazi, A. Mpimbaza, J.P. Mpindi, M. Musinguzi, G. Musoke, W. Musoke, K. Mwebaze, R. Nakafero, R. Namunyiga, J. Nankabirwa, D. Njama, S. Nsobya, R. Oluga, R. Osaliya, R. Rarigye, C. Tuganineyo, N.L. Wilson, and P.B. Yakika. Uganda Transfusion Study: C. Aziku, F. Banage, H. Kyomugisha, R. Pade, D. Mimbe,E. Mwesigye, G. Luganda,G. Nabukeera, J. Namugga, A.P. Musinguzi, S. Kulabako, R. Nalumansi, J. Nansubuga, J. Ngabirwe, A. Owor, and I. Tumuheirwe. We also acknowledge Dr. David Scadden for assisting in granting access to these communities.
Financial support: National Institutes of Health (K01 HD052020, T32 MH19105, U01 AI052142, R01 CA119903, and HHSN261200800001E), Gladstone Institute of Virology and Immunology-University of California, San Francisco Center for AIDS Research (P30 AI027763), AIDS Malignancy Consortium (U01 CA078124), University of California Universitywide AIDS Research Program (CC99-SF-001), South Africa National Research Foundation (Thuthuka Programme Grant 2054349), Doris Duke Charitable Foundation (to P.J.R. and G.D.), AIDS Care Research in Africa Program, and Centers for Disease Control and Prevention.
Footnotes
Potential conflicts of interest: none reported.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of Centers for Disease Control and Prevention or the US Department of Health and Human Services.
References
- 1.Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–9. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Martin JN, Ganem DE, Osmond DH, Page-Shafer KA, Macrae D, Kedes DH. Sexual transmission and the natural history of human herpesvirus 8 infection. N Engl J Med. 1998;338:948–54. doi: 10.1056/NEJM199804023381403. [DOI] [PubMed] [Google Scholar]
- 3.O'Brien TR, Kedes D, Ganem D, et al. Evidence for concurrent epidemics of human herpesvirus 8 and human immunodeficiency virus type 1 in US homosexual men: rates, risk factors, and relationship to Kaposi's sarcoma. J Infect Dis. 1999;180:1010–7. doi: 10.1086/315039. [DOI] [PubMed] [Google Scholar]
- 4.Whitby D, Howard MR, Tenant-Flowers M, et al. Detection of Kaposi sarcoma associated herpesvirus in peripheral blood of HIV-infected individuals and progression to Kaposi's sarcoma. Lancet. 1995;346:799–802. doi: 10.1016/s0140-6736(95)91619-9. [DOI] [PubMed] [Google Scholar]
- 5.Gao SJ, Kingsley L, Hoover DR, et al. Seroconversion to antibodies against Kaposi's sarcoma-associated herpesvirus-related latent nuclear antigens before the development of Kaposi's sarcoma. N Engl J Med. 1996;335:233–41. doi: 10.1056/NEJM199607253350403. [DOI] [PubMed] [Google Scholar]
- 6.Renwick N, Halaby T, Weverling GJ, et al. Seroconversion for human herpesvirus 8 during HIV infection is highly predictive of Kaposi's sarcoma. AIDS. 1998;12:2481–8. doi: 10.1097/00002030-199818000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Melbye M, Cook PM, Hjalgrim H, et al. Risk factors for Kaposi's-sarcoma-associated herpesvirus (KSHV/HHV-8) seropositivity in a cohort of homosexual men, 1981–1996. Int J Cancer. 1998;77:543–8. doi: 10.1002/(sici)1097-0215(19980812)77:4<543::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Kedes DH, Ganem D, Ameli N, Bacchetti P, Greenblatt R. The prevalence of serum antibody to human herpesvirus 8 (Kaposi sarcoma-associated herpesvirus) among HIV-seropositive and high-risk HIV-seronegative women. JAMA. 1997;277:478–81. [PubMed] [Google Scholar]
- 9.Dukers NH, Rezza G. Human herpesvirus 8 epidemiology: what we do and do not know. AIDS. 2003;17:1717–30. doi: 10.1097/01.aids.0000076337.42412.86. [DOI] [PubMed] [Google Scholar]
- 10.Smith NA, Sabin CA, Gopal R, et al. Serologic evidence of human herpesvirus 8 transmission by homosexual but not heterosexual sex. J Infect Dis. 1999;180:600–6. doi: 10.1086/314926. [DOI] [PubMed] [Google Scholar]
- 11.Chandran B, Smith MS, Koelle DM, Corey L, Horvat R, Goldstein E. Reactivities of human sera with human herpesvirus-8-infected BCBL-1 cells and identification of HHV-8-specific proteins and glycoproteins and the encoding cDNAs. Virology. 1998;243:208–17. doi: 10.1006/viro.1998.9055. [DOI] [PubMed] [Google Scholar]
- 12.Belec L, Cancre N, Hallouin MC, Morvan J, Si Mohamed A, Gresenguet G. High prevalence in Central Africa of blood donors who are potentially infectious for human herpesvirus 8. Transfusion. 1998;38:771–5. doi: 10.1046/j.1537-2995.1998.38898375517.x. [DOI] [PubMed] [Google Scholar]
- 13.Mbulaiteye SM, Biggar RJ, Pfeiffer RM, et al. Water, socioeconomic factors, and human herpesvirus 8 infection in Ugandan children and their mothers. J Acquir Immune Defic Syndr. 2005;38:474–9. doi: 10.1097/01.qai.0000132495.89162.c0. [DOI] [PubMed] [Google Scholar]
- 14.Baeten JM, Chohan BH, Lavreys L, et al. Correlates of human herpesvirus 8 seropositivity among heterosexual men in Kenya. AIDS. 2002;16:2073–8. doi: 10.1097/00002030-200210180-00013. [DOI] [PubMed] [Google Scholar]
- 15.Kakoola DN, Sheldon J, Byabazaire N, et al. Recombination in human herpesvirus-8 strains from Uganda and evolution of the K15 gene. J Gen Virol. 2001;82:2393–404. doi: 10.1099/0022-1317-82-10-2393. [DOI] [PubMed] [Google Scholar]
- 16.Hladik W, Dollard SC, Downing RG, et al. Kaposi's sarcoma in Uganda: risk factors for human herpesvirus 8 infection among blood donors. J Acquir Immune Defic Syndr. 2003;33:206–10. doi: 10.1097/00126334-200306010-00015. [DOI] [PubMed] [Google Scholar]
- 17.Wawer MJ, Eng SM, Serwadda D, et al. Prevalence of Kaposi sarcoma–associated herpesvirus compared with selected sexually transmitted diseases in adolescents and young adults in rural Rakai District, Uganda. Sex Transm Dis. 2001;28:77–81. doi: 10.1097/00007435-200102000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Eltom MA, Mbulaiteye SM, Dada AJ, Whitby D, Biggar RJ. Transmission of human herpesvirus 8 by sexual activity among adults in Lagos, Nigeria. AIDS. 2002;16:2473–8. doi: 10.1097/00002030-200212060-00014. [DOI] [PubMed] [Google Scholar]
- 19.Plancoulaine S, Abel L, Tregouet D, et al. Respective roles of serological status and blood specific antihuman herpesvirus 8 antibody levels in human herpesvirus 8 intrafamilial transmission in a highly endemic area. Cancer Res. 2004;64:8782–7. doi: 10.1158/0008-5472.CAN-04-2000. [DOI] [PubMed] [Google Scholar]
- 20.Serraino D, Toma L, Andreoni M, et al. A seroprevalence study of human herpesvirus type 8 (HHV8) in eastern and Central Africa and in the Mediterranean area. Eur J Epidemiol. 2001;17:871–6. doi: 10.1023/a:1015678312153. [DOI] [PubMed] [Google Scholar]
- 21.Bourboulia D, Whitby D, Boshoff C, et al. Serologic evidence for mother-to-child transmission of Kaposi sarcoma–associated herpesvirus infection. JAMA. 1998;280:31–2. doi: 10.1001/jama.280.1.31-a. [DOI] [PubMed] [Google Scholar]
- 22.Malope BI, Pfeiffer RM, Mbisa G, et al. Transmission of Kaposi sarcoma–associated herpesvirus between mothers and children in a South African population. J Acquir Immune Defic Syndr. 2007;44:351–5. doi: 10.1097/QAI.0b013e31802f12ea. [DOI] [PubMed] [Google Scholar]
- 23.Dedicoat M, Newton R, Alkharsah KR, et al. Mother-to-child transmission of human herpesvirus-8 in South Africa. J Infect Dis. 2004;190:1068–75. doi: 10.1086/423326. [DOI] [PubMed] [Google Scholar]
- 24.Minhas V, Crabtree KL, Chao A, et al. Early childhood infection by human herpesvirus 8 in Zambia and the role of human immunodeficiency virus type 1 coinfection in a highly endemic area. Am J Epidemiol. 2008;168:311–20. doi: 10.1093/aje/kwn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martro E, Bulterys M, Stewart JA, et al. Comparison of human herpesvirus 8 and Epstein-Barr virus seropositivity among children in areas endemic and non-endemic for Kaposi's sarcoma. J Med Virol. 2004;72:126–31. doi: 10.1002/jmv.10548. [DOI] [PubMed] [Google Scholar]
- 26.Enbom M, Tolfvenstam T, Ghebrekidan H, et al. Seroprevalence of human herpes virus 8 in different Eritrean population groups. J Clin Virol. 1999;14:167–72. doi: 10.1016/s1386-6532(99)00061-x. [DOI] [PubMed] [Google Scholar]
- 27.Gessain A, Mauclere P, van Beveren M, et al. Human herpesvirus 8 primary infection occurs during childhood in Cameroon, Central Africa. Int J Cancer. 1999;81:189–92. doi: 10.1002/(sici)1097-0215(19990412)81:2<189::aid-ijc4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Mayama S, Cuevas LE, Sheldon J, et al. Prevalence and transmission of Kaposi's sarcoma–associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int J Cancer. 1998;77:817–20. doi: 10.1002/(sici)1097-0215(19980911)77:6<817::aid-ijc2>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 29.Mbulaiteye SM, Biggar RJ, Bakaki PM, et al. Human herpesvirus 8 infection and transfusion history in children with sickle-cell disease in Uganda. J Natl Cancer Inst. 2003;95:1330–5. doi: 10.1093/jnci/djg039. [DOI] [PubMed] [Google Scholar]
- 30.Mbulaiteye SM, Pfeiffer RM, Whitby D, Brubaker GR, Shao J, Biggar RJ. Human herpesvirus 8 infection within families in rural Tanzania. J Infect Dis. 2003;187:1780–5. doi: 10.1086/374973. [DOI] [PubMed] [Google Scholar]
- 31.Rabkin CS, Schulz TF, Whitby D, et al. Interassay correlation of human herpesvirus 8 serologic tests. J Infect Dis. 1998;178:304–9. doi: 10.1086/515649. [DOI] [PubMed] [Google Scholar]
- 32.Cook-Mozaffari P, Newton R, Beral V, Burkitt DP. The geographical distribution of Kaposi's sarcoma and of lymphomas in Africa before the AIDS epidemic. Br J Cancer. 1998;78:1521–8. doi: 10.1038/bjc.1998.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis JC, Clark TD, Kemble SK, et al. Longitudinal study of urban malaria in a cohort of Ugandan children: description of study site, census and recruitment. Malar J. 2006;5:18. doi: 10.1186/1475-2875-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hladik W, Dollard SC, Mermin J, et al. Transmission of human herpesvirus 8 by blood transfusion. N Engl J Med. 2006;355:1331–8. doi: 10.1056/NEJMoa055009. [DOI] [PubMed] [Google Scholar]
- 35.Pau CP, Lam LL, Spira TJ, et al. Mapping and serodiagnostic application of a dominant epitope within the human herpesvirus 8 ORF 65–encoded protein. J Clin Microbiol. 1998;36:1574–7. doi: 10.1128/jcm.36.6.1574-1577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dollard SC, Nelson KE, Ness PM, et al. Possible transmission of human herpesvirus-8 by blood transfusion in a historical United States cohort. Transfusion. 2005;45:500–3. doi: 10.1111/j.0041-1132.2005.04334.x. [DOI] [PubMed] [Google Scholar]
- 37.Pellett PE, Wright DJ, Engels EA, et al. Multicenter comparison of serologic assays and estimation of human herpesvirus 8 seroprevalence among US blood donors. Transfusion. 2003;43:1260–8. doi: 10.1046/j.1537-2995.2003.00490.x. [DOI] [PubMed] [Google Scholar]
- 38.Cannon MJ, Operskalski EA, Mosley JW, Radford K, Dollard SC. Lack of evidence for human herpesvirus-8 transmission via blood transfusion in a historical US cohort. J Infect Dis. 2009;199:1592–8. doi: 10.1086/598859. [DOI] [PubMed] [Google Scholar]
- 39.Malope BI, MacPhail P, Mbisa G, et al. No evidence of sexual transmission of Kaposi's sarcoma herpes virus in a heterosexual South African population. AIDS. 2008;22:519–26. doi: 10.1097/QAD.0b013e3282f46582. [DOI] [PubMed] [Google Scholar]
- 40.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–30. [PubMed] [Google Scholar]
- 41.Mantina H, Kankasa C, Klaskala W, et al. Vertical transmission of Kaposi's sarcoma–associated herpesvirus. Int J Cancer. 2001;94:749–52. doi: 10.1002/ijc.1529. [DOI] [PubMed] [Google Scholar]
- 42.Chohan BH, Taylor H, Obrigewitch R, et al. Human herpesvirus 8 seroconversion in Kenyan women by enzyme-linked immunosorbent assay and immunofluorescence assay. J Clin Virol. 2004;30:137–44. doi: 10.1016/j.jcv.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 43.Hayward GS. KSHV strains: the origins and global spread of the virus. Semin Cancer Biol. 1999;9:187–99. doi: 10.1006/scbi.1998.0116. [DOI] [PubMed] [Google Scholar]
- 44.Andreoni M, Sarmati L, Nicastri E, et al. Primary human herpesvirus 8 infection in immunocompetent children. JAMA. 2002;287:1295–300. doi: 10.1001/jama.287.10.1295. [DOI] [PubMed] [Google Scholar]
- 45.Avelleira JC, Lupi O, Caterino-de-Araujo A, Santos-Fortuna Ede L. Seroprevalence of HHV-8 infection in the pediatric population of two university hospitals in Rio de Janeiro, Brazil. Int J Dermatol. 2006;45:381–3. doi: 10.1111/j.1365-4632.2006.02523.x. [DOI] [PubMed] [Google Scholar]
- 46.Lyall EG, Patton GS, Sheldon J, et al. Evidence for horizontal and not vertical transmission of human herpesvirus 8 in children born to human immunodeficiency virus–infected mothers. Pediatr Infect Dis J. 1999;18:795–9. doi: 10.1097/00006454-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Alkharsah KR, Dedicoat M, Blasczyk R, Newton R, Schulz TF. Influence of HLA alleles on shedding of Kaposi sarcoma–associated herpesvirus in saliva in an African population. J Infect Dis. 2007;195:809–16. doi: 10.1086/511827. [DOI] [PubMed] [Google Scholar]
- 48.Plancoulaine S, Gessain A, van Beveren M, Tortevoye P, Abel L. Evidence for a recessive major gene predisposing to human herpesvirus 8 (HHV-8) infection in a population in which HHV-8 is endemic. J Infect Dis. 2003;187:1944–50. doi: 10.1086/375345. [DOI] [PubMed] [Google Scholar]
- 49.Coluzzi M, Calabro ML, Manno D, Chieco-Bianchi L, Schulz TF, Ascoli V. Saliva and the transmission of human herpesvirus 8: potential role of promoter-arthropod bites. J Infect Dis. 2004;190:199–200. doi: 10.1086/420890. author reply 200−1. [DOI] [PubMed] [Google Scholar]
- 50.Whitby D, Marshall VA, Bagni RK, et al. Reactivation of Kaposi's sarcoma–associated herpesvirus by natural products from Kaposi's sarcoma endemic regions. Int J Cancer. 2007;120:321–8. doi: 10.1002/ijc.22205. [DOI] [PMC free article] [PubMed] [Google Scholar]