Abstract
Anterior gradient 2 (AGR2) is a secreted, cancer-associated protein in many types of epithelial cancer cells. We developed a highly sensitive targeted mass spectrometric assay for quantification of AGR2 in urine and serum. Digested peptides from clinical samples were processed by PRISM (high pressure and high resolution separations coupled with intelligent selection and multiplexing), which incorporates high pH reversed-phase LC separations to fractionate and select target fractions for follow-on LC-SRM analyses. The PRISM-SRM assay for AGR2 showed a reproducibility of <10% CV and LOQ values of ~130 pg/mL in serum and ~10 pg per 100 μg total protein mass in urine, respectively. A good correlation (R2 = 0.91) was observed for the measurable AGR2 concentrations in urine between SRM and ELISA. Based on an initial cohort of 37 subjects, urinary AGR2/PSA concentration ratios showed a significant difference (P = 0.026) between non-cancer and cancer. Large clinical cohort studies are needed for the validation of AGR2 as a useful diagnostic biomarker for prostate cancer. Our work validated the approach of identifying candidate secreted protein biomarkers through genomics and measurement by targeted proteomics, especially for proteins where no immunoassays are available.
Keywords: AGR2, PSA, prostate cancer, PRISM-SRM, human urine, human serum
INTRODUCTION
Prostate cancer is the most common cancer in men in the US with 241,740 new cases and 28,170 prostate cancer-related deaths in 2012.1 Prostate cancer ranks as the second leading cause of cancer death in men and accounts for 29% of all male cancers and 9% of male cancer-related deaths.2 One standard clinical test that measures the level of prostate-specific antigen (PSA) in blood is currently being used for early detection of this cancer. However, serum PSA is an imperfect biomarker with an inherent flaw because PSA, although prostate specific, is not cancer-specific. Blood PSA levels can be increased by causes not related to cancer.3 Thus, PSA screening results in many unnecessary biopsies of men showing abnormal PSA levels, and misses some men with normal PSA levels but harboring cancer. Recently, two prostate cancer-specific urinary tests for measuring PCA3 and TMPRSS2:ERG fusion transcript levels were introduced for detecting cancer cells presumably released into the urine by an attentive digital rectal exam.4 Compared with serum PSA, PCA3 shows higher specificity but lower sensitivity.5 For TMPRSS2:ERG, the principal drawback is that this fusion event is absent in 40–50% of the Caucasian cancer cases.6 There remains an urgent need to develop new specific molecular biomarkers for early diagnosis and means to better stratify prostate cancer patients for treatment.
Recent studies have shown that anterior gradient 2 (AGR2) is a potentially useful urine biomarker for prostate cancer.7–9 AGR2 is a secreted, cancer-associated protein and upregulated in many types of epithelial cancer,10,11 including breast,12,13 lung,14,15 colon,16 ovarian,17,18 esophageal,19 and pancreatic20,21 beside prostate. Compared with non-cancer, AGR2 is highly expressed in prostate cancer at both the mRNA and protein levels.8,9 In particular, good performance of urinary AGR2/PSA transcript ratio has been recently demonstrated for differentiating prostate cancer patients from non-cancer.8 Therefore, there is a need to assess the potential utility of AGR2 in urine or blood as a cancer biomarker.
Liquid chromatography (LC)-selected reaction monitoring (SRM) has recently emerged as a promising alternative to immunoassays for protein quantification due to its high specificity and multiplexing capability.22,23 Immunoassay development is time consuming involving the generation of highly specific monoclonal antibodies. A major limitation of typical LC-SRM analysis is the insufficient sensitivity to detect low-abundance protein biomarkers in body fluids (e.g., less than 1 ng/mL in blood plasma/serum), cells or tissues.22 To address this issue, sample prefractionation24 and/or immunoaffinity enrichment strategies25,26 have been implemented to enhance SRM sensitivity. Recently, we developed an antibody-free, targeted mass spectrometric approach termed high pressure and high resolution separations coupled with intelligent selection and multiplexing (PRISM) for highly sensitive quantification of serum proteins at the sub-ng/mL levels.27 To enable validation of AGR2 as a potential cancer biomarker, we aimed to develop a reliable and highly sensitive PRISM-SRM assay for quantification of AGR2 at low pg/mL levels in both urine and serum samples in this study.
EXPERIMENTAL PROCEDURES
Reagents
Urea, dithiothreitol (DTT), iodoacetamide, ammonium formate, trifluoroacetic acid (TFA) and formic acid were purchased from Sigma (St. Louis, MO). The synthetic peptides labeled with 13C/15N on C-terminal lysine and arginine residues were from Thermo Scientific (San Jose, CA). The heavy peptides for PSA protein were estimated to be of >95% purity by HPLC and the purity of crude heavy peptides for AGR2 (UniProt ID: Q4JM46) was not known. Recombinant AGR2 (>95% purity) used for generating calibration curves was purchased from GenWay Biotech, Inc (San Diego, CA).
Human Specimens
The use of human urine and blood serum samples was approved by the Institutional Review Boards of the University of Washington, Pacific Northwest National Laboratory, University of Texas Health Science Center at San Antonio, and Johns Hopkins University in accordance with federal regulations. The urine samples were collected from consented pre-operative patients (incident cases), and their tumor characteristics were described in a previous report.9 The control specimens were obtained from healthy men with no cancer diagnosis (normal PSA and/or DRE finding). Donors were recruited with written consent. Clinical serum samples were obtained from patients undergoing PSA testing for prostate cancer at UT San Antonio and were analyzed in a CLIA certified laboratory at Johns Hopkins.
Urine and Serum Sample Processing and Digestion
Voided urine samples were collected and usually processed within 2 h. The urine samples were partitioned into supernatant for protein analysis and sediment for RNA analysis by centrifugation at 1,000 g for 5 min. The supernatant was stored at −80° C. There was generally insufficient protein material from the sediment for PRISM-SRM measurement. Fifteen mL of urine were desalted and concentrated using Amicon® Ultra-15 (3 kDa nominal molecular weight cut-off, Millipore, Billerica, MA).28 Protein concentrations were determined by the BCA assay (Pierce, Rockford, IL). Concentrated urinary proteins from each sample, ranging from 200 to 300 μg, were denatured and reduced with 8 M urea and 10 mM DTT in 50 mM NH4HCO3, pH 8.0 for 1 h at 37° C. Protein cysteine residues were alkylated with 40 mM iodoacetamide for 1 h at room temperature in the dark. The sample was diluted 6-fold with 50 mM NH4HCO3, pH 8.0, and digested by sequencing-grade modified porcine trypsin (Promega, Madison, WI) with a 1:50 trypsin:protein ratio (w/w) at 37 °C overnight. The resulting digest was then desalted by using a 1 mL SPE C18 column (Supelco, Bellefonte, PA) as described previously.29 The final peptide concentration was determined by BCA. The peptide sample was diluted to 0.5 μg/μL with 0.1% formic acid in water, and heavy isotope-labeled synthetic peptides at an equimolar concentration (approximately 10 fmol/μL of crude heavy peptides for AGR2 and 0.5 fmol/μL of high purity heavy peptides for PSA) were spiked in.
For prostate cancer patient serum samples, 125 μL of serum was first loaded onto IgY14 immunoaffinity column to remove 14 high-abundance proteins as described previously.27 The IgY14 flow-through proteins were then digested using the same protocol as described above for the urine samples.
Generation of Calibration Curve Using Recombinant AGR2
Ten μg of recombinant AGR2 were digested as described above. The digested AGR2 was added to a digested urine peptide sample (with a concentration of 20 ng/μL) at 0, 0.1, 0.25, 0.5, 1, 2.5, 5, 7.5, 10, 50, 100 and 500 pg/μL levels. Heavy synthetic crude peptides were also spiked into each sample at a concentration of approximately 100 fmol/μL and the final sample preparations were analyzed by LC-SRM. The generated AGR2 calibration curves were only used for calculating the endogenous AGR2 protein concentrations in patient urine or sera, not for determining the LOD and LOQ (the diluted urine was used as the matrix to reduce sample loss because all our urine samples have certain amounts of AGR2 protein).
SRM Assay Configuration
Ten tryptic surrogate peptides were initially chosen for AGR2 based upon in silico trypsin digestion and existing MS/MS data from our laboratory and the Global Proteome Machine (GPM). These peptides were then evaluated by ESP predictor30 and CONSeQuence31 software. Four peptides with moderate hydrophobicity and high scores from the prediction tools, LAEQFVLLNLVYETTDK, GWGDQLIWTQTYEEALYK, HLSPDGQYVPR, LPQTLSR, were selected for peptide synthesis. The synthesized crude heavy-isotope labeled peptides were further evaluated for peptide response and fragmentation pattern. Optimal collision energy (CE) values were achieved by direct infusion of the individual peptides and/or multiple LC-SRM runs with CE ramping. Two best performing peptides, HLSPDGQYVPR and LPQTLSR, were selected for detection and quantification of AGR2. For each peptide, three best transitions were selected, and matrix interference was determined. Briefly, the relative intensity ratios among the three final selected transitions for the SRM assay were predefined by internal standard heavy peptides in a buffer. Matrix interference for a given transition that fell into the mass width of Q1 and Q3 from co-eluting peptides was determined by deviation from the expected relative intensity ratios between the transitions. While three transitions per peptide were monitored, the best transition with no matrix interference was used for generating the calibration curve and AGR2 quantification in clinical urine and serum samples. We observed that the use of best transition with no matrix interference for quantification while monitoring multiple transitions per peptide provides the best specificity and sensitivity.
PRISM-SRM Measurement
The PRISM-SRM approach has been previously described for quantification of low-abundance proteins in human plasma or serum.27 Briefly, high resolution reversed phase capillary LC with pH 10 mobile phase was used as the first dimensional separation of peptides from trypsin-digested human urine or serum (Figure S1). Following cLC separation, the column eluent was automatically collected every minute into a 96-well plate during a ~100 min LC run while on-line SRM monitoring of heavy internal standard peptides was performed from a small stream of the flow. Intelligent selection (termed iSelection) of target peptide fractions was achieved based on the on-line SRM signal of internal standard peptides. Prior to peptide fraction collection, 17 μL of water was added to each well of the 96-well plate to avoid loss of peptides and dilute the peptide fractions (nearly 1:7 dilution) for LC-SRM analysis.
Following iSelection, the AGR2 target peptide-containing fractions were subjected to LC-SRM measurement (Figure S1). All peptide fractions were analyzed by using nanoACQUITY UPLC® system (Waters Corporation, Milford, MA) coupled on-line to a TSQ Vantage triple quadrupole mass spectrometer (Thermo Scientific, San Jose, CA). Solvents used were 0.1% formic acid in water (mobile phase A) and 0.1% formic acid in 90% acetonitrile (mobile phase B). Peptide separations were performed at a mobile phase flow rate of 400 nL/min using an ACQUITY UPLC BEH 1.7 μm C18 column (75 μm i.d. × 10 cm), which was connected to a chemically etched 20 μm i.d. fused-silica emitter via a Valco stainless steel union. Either 1 μL of unfractionated urine digests or 4 μL of individual peptide fractions (total volume 20 μL) following PRISM was injected for LC separations using a binary gradient of 10–20% B in 7 min, 20–25% B in 17 min, 25–40% B in 1.5 min, 40–95% B in 2.5 min and 95% B for 6 min for a total of ~35 min. The TSQ Vantage was operated in the same manner as previously described.27 The scan width of 0.002 m/z and a dwell time of 40 ms were set for all SRM transitions.
Data Analysis
SRM data acquired on the TSQ Vantage were analyzed using Xcalibur 2.0.7 (Thermo Scientific). Peak detection and integration were determined based on two criteria: 1) same retention time; 2) approximately same relative SRM peak intensity ratios across multiple transitions between light peptides and heavy peptide standards. All data were manually inspected to ensure correct peak detection and accurate integration. The L/H SRM peak area ratio was used to generate the calibration curves and assess reproducibility. The RAW data from TSQ Vantage were loaded into Skyline software32 to create high resolution figures of extracted ion chromatograms (XIC) of multiple transitions monitored for the target proteins. GraphPad Prism 6.0 was used for statistical analysis and plotting and P < 0.05 was considered significant.
RESULTS
Peptide Selection
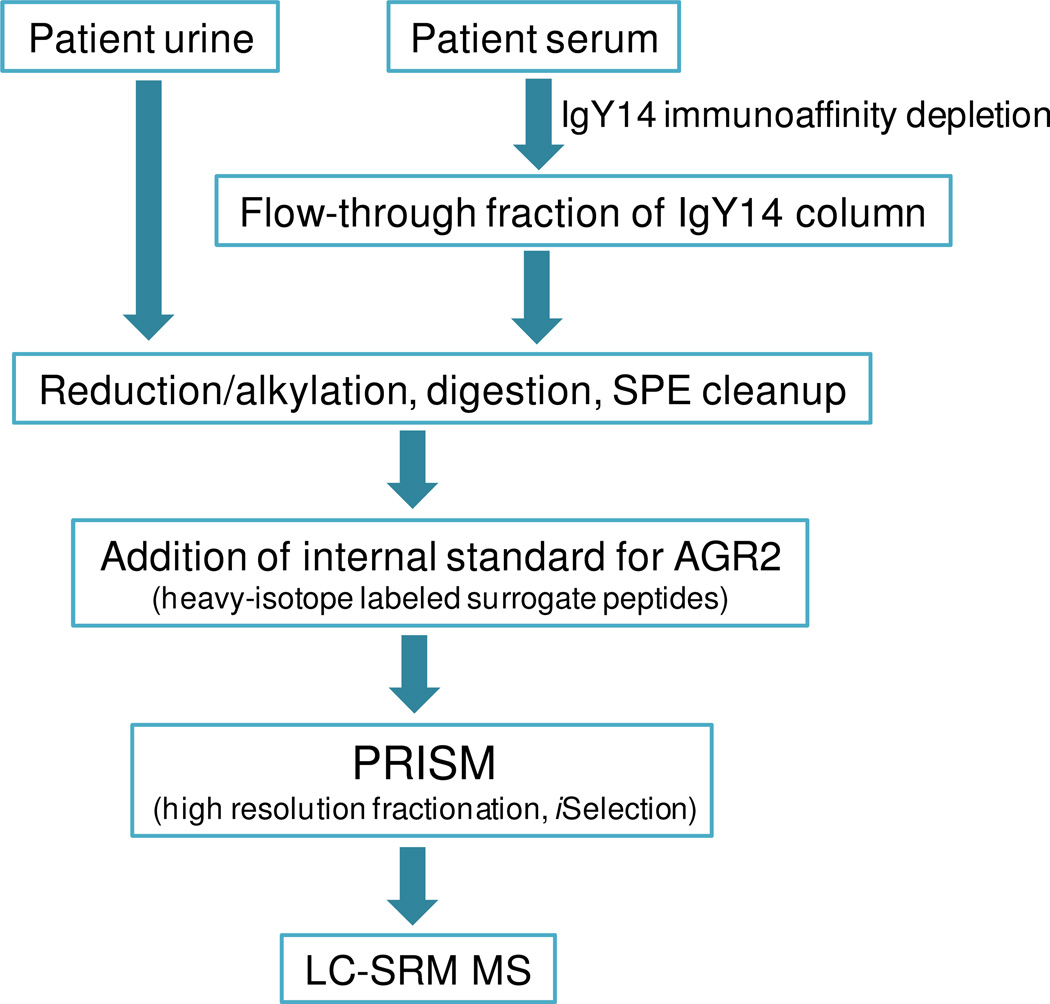
The best performing peptides identified were HLSPDGQYVPR and LPQTLSR for quantification of AGR2. For PSA, IVGGWECcamEK (Ccam: cysteine residues synthesized as carbamidomethyl cysteine) and LSEPAELTDAVK were selected based on previous study results as the most effective in quantification.24,27 The labeled synthetic peptides were added to test samples (urine and serum) for SRM analysis as shown in Figure 1.
Figure 1.
PRISM-SRM workflow for sensitive detection and quantification of AGR2 in urine and serum.
Calibration Curves of the AGR2 SRM Assay
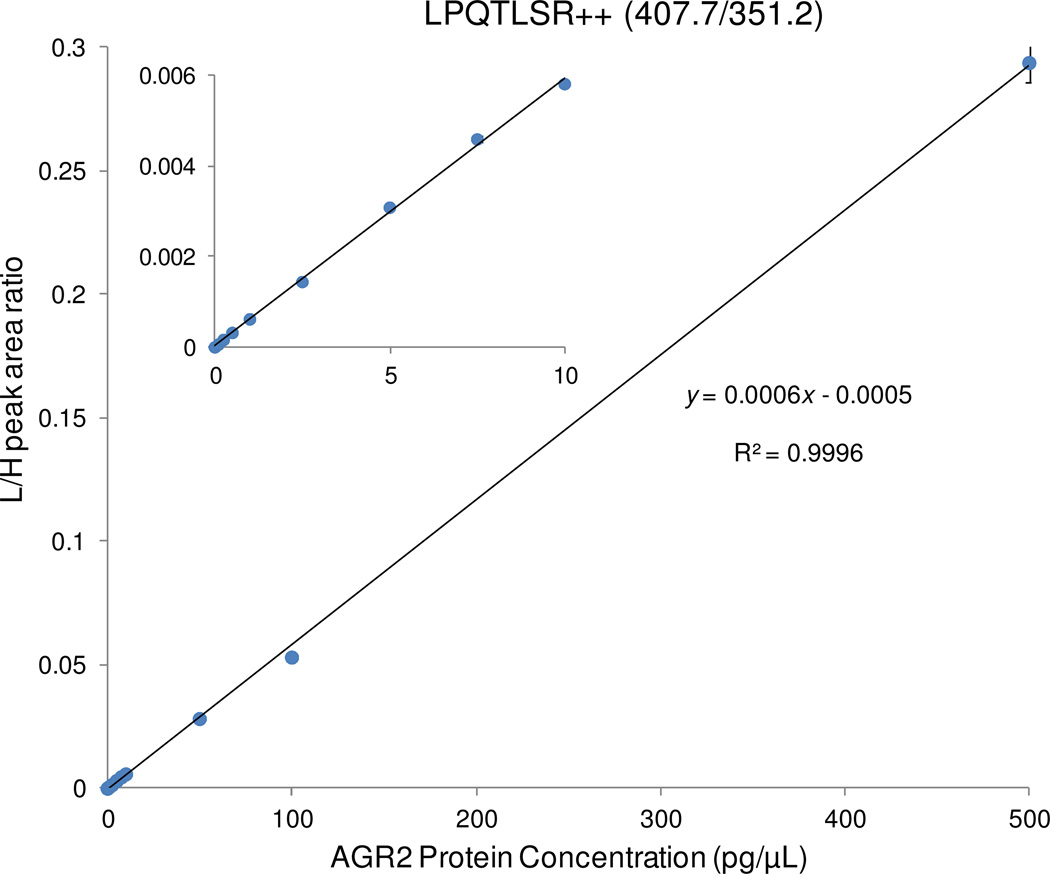
To quantify AGR2 concentrations in urine and serum, a calibration curve was generated by spiking in tryptic digest of recombinant AGR2 protein at various concentrations to the same diluted urine matrix (25-fold dilution) containing crude heavy isotope-labeled synthetic peptides as internal standards. The calibration curve was used only to determine the endogenous AGR2 concentrations rather than the limit of detection (LOD) and limit of quantification (LOQ). Figure 2 shows the calibration curve from the best transition, 407.7/351.2, where an excellent linearity for AGR2 concentrations ranging from 0.1 pg/μL to 500 pg/μL was observed with a median coefficient of variation (CV) of ~2.8% in triplicate LC-SRM measurements (Table S1).
Figure 2.
Calibration curve for quantifying AGR2 using diluted urine (20 ng/μL) as the matrix. Recombinant AGR2 with a concentration range from 0.1 to 500 pg/μL was spiked into the diluted urine. The inset plot shows details of the low concentration points. Except for the high concentration point, the error bars of the other concentration points are smaller than the size of the points (Table S1).
PRISM-SRM Measurements of AGR2 in Urine and Serum Samples
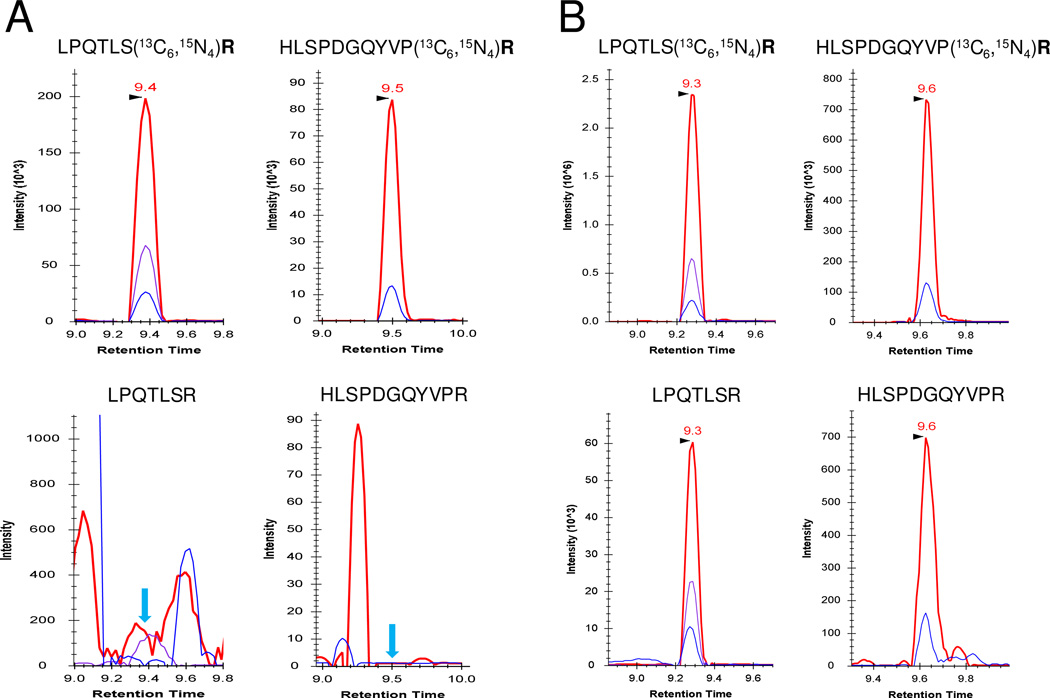
A conventional LC-SRM assay was explored first to detect AGR2 in prostate cancer patient urine (Table S2). Both surrogate peptides of AGR2 were not detectable due to heavy matrix interference (Figure 3). However, when PRISM-SRM was applied, both AGR2 endogenous light peptides were readily detectable and quantifiable in all 37 urine samples [14 pre-operative cancer (CP) and 23 non-cancer (NP) samples]. Figures 3A and 3B show one example of XIC of transitions monitored for the two AGR2 surrogate peptides with and without the application of PRISM. Clearly, PRISM enabled sensitive detection of AGR2 in the urine due to the enrichment of targeted peptides and dramatically reduced background interference.
Figure 3.
Extracted ion chromatograms (XIC) of transitions monitored for AGR2 signature peptides LPQTLSR and HLSPDGQYVPR in a prostate cancer urine sample (A) without PRISM; (B) with PRISM. LPQTLSR: 407.7/351.2 (red), 407.7/476.3 (purple), 407.7/604.3 (blue); HLSPDGQYVPR: 634.8/1018.5 (red), 634.8/1131.6 (blue). Internal standards (top XIC) were spiked at10 fmol/μL. The blue arrows in (A) indicate the locations of expected SRM peak apices of light peptides (bottom XIC) based on the retention time of heavy internal standards.
PRISM-SRM was also applied for quantification of AGR2 levels in a set of serum samples collected from prostate cancer patients with known blood PSA levels (measured by ELISA). The abundance of AGR2 in blood has been reported to be very low, less than 1 ng/mL level in human serum and beyond detection by our current AGR2 ELISA assay,9 though others have reported a sensitive AGR2 ELISA assay at detecting 100 pg/mL levels of AGR2 protein in the blood of ovarian cancer patients.18 That ELISA, unlike ours based on two monoclonal antibodies, used a monoclonal and a rabbit polyclonal. Polyclonals are by nature not as stringent as monoclonals. Therefore, IgY14 immunoaffinity depletion was applied prior to PRISM to provide sufficient sensitivity for PRISM-SRM detection of AGR2 in human serum. SRM signals of light peptides derived from the endogenous serum AGR2 were confidently detected in patient sera with a signal-to-noise ratio (S/N) >3 (Figure S2).
Quantification of AGR2 Concentration
With the established calibration curve and the measured L/H peak area ratios for individual samples, the AGR2 concentration in each sample could be calculated and expressed as pg/100 μg total protein mass for urine and pg/mL for serum (Supplemental Methods, Supporting Information). The L/H peak area ratios and the calculated AGR2 concentrations in prostate cancer patient urine are summarized in Table 1. For serum AGR2 concentrations, however, the calibration curve of the transition, 407.7/604.3, from LPQTLSR was used because matrix interference was observed for the best transition, 407.7/351.2 (Figure S2). Except one patient serum sample with an S/N ratio lower than 10, the AGR2 protein concentration of the other clinical serum samples could be accurately quantified using PRISM-SRM. A correlation between AGR2 levels and PSA concentrations was observed for the 5 patient serum samples tested (Figure S4), suggesting that AGR2 was potentially associated with the presence of prostate cancer. More clinical samples are being collected to demonstrate this correlation between AGR2 and PSA protein concentrations in sera.
Table 1.
Summary of PRISM-SRM measurements of 37 urine samples
| Prostate cancer patient urine Ida |
L/Hb | CV | AGR2 concentration (pg/100 μg urinary protein) | ||
|---|---|---|---|---|---|
| PRISM-SRM | ELISAc | ||||
| Non-cancer | P07022BN | 0.00172 | 5.9% | 55.5 | |
| P07036BN | 0.00375 | 7.1% | 127.0 | ||
| P08018BN | 0.00077 | 13.5% | 26.6 | ||
| P08022BN | 0.00290 | 2.3% | 96.5 | ||
| P08036BN | 0.00197 | 10.5% | 64.7 | ||
| P09040AN | 0.00780 | 5.5% | 305.3 | ||
| SAP12_003BN | 0.00410 | 10.8% | 137.4 | ||
| SAP12_008BN | 0.00735 | 5.3% | 251.9 | ||
| SAP12_014BN | 0.00320 | 5.8% | 106.8 | ||
| SAP12_016BN | 0.00435 | 7.6% | 144.6 | ||
| SAP12_017BN | 0.00610 | 4.9% | 207.1 | ||
| SAP12_026BN | 0.02220 | 2.1% | 805.7 | ||
| SAP12_028BN | 0.00570 | 3.9% | 193.0 | ||
| SAP12_030BN | 0.00295 | 12.4% | 97.1 | ||
| SAP12_033BN | 0.00315 | 15.1% | 105.1 | ||
| SAP12_034BN | 0.00500 | 6.7% | 168.0 | ||
| SAP12_035BN | 0.01040 | 2.6% | 361.2 | ||
| SAP12_039BN | 0.01230 | 3.1% | 429.3 | ||
| SAP12_050BN | 0.01790 | 2.3% | 630.0 | ||
| SAP12_052BN | 0.00690 | 11.5% | 235.7 | ||
| SAP12_071BN | 0.00790 | 7.5% | 271.6 | ||
| SAP12_072BN | 0.00315 | 12.3% | 105.1 | ||
| SAP12_077BN | 0.00630 | 5.9% | 214.2 | ||
| Cancer | P06003Pre | 0.00037 | 1.4% | 12.1 | |
| P06011Pre | 0.00333 | 15.1% | 112.0 | 21 | |
| P06017Pre | 0.00339 | 16.5% | 114.2 | ||
| P07016Pre | 0.00576 | 8.5% | 263.4 | ||
| P07018Pre | 0.01228 | 9.8% | 494.1 | 78 | |
| P07019Pre | 0.00086 | 7.1% | 30.7 | ||
| P07029Pre | 0.00011 | 15.6% | 3.0 | ||
| P07031Pre | 0.02681 | 16.6% | 963.3 | 208 | |
| P07040Pre | 0.00395 | 14.2% | 120.5 | ||
| P07047Pre | 0.00073 | 15.2% | 23.5 | 2.3 | |
| P08006rPre | 0.00133 | 4.2% | 48.8 | ||
| P08015Pre | 0.00233 | 4.6% | 69.8 | 62.5 | |
| P08028Pre | 0.00206 | 13.3% | 68.5 | ||
| P08032Pre | 0.00086 | 2.3% | 30.8 | 10 | |
Clinical prostate cancer patient and non-cancer urine samples.
Average L/H peak area ratios for three LC-SRM measurements.
AGR2 concentration measured by ELISA.
Precision and LOQ of the AGR2 Assay
The reproducibility of AGR2 SRM assay was assessed by process replicates, where five identical aliquots from the same urine samples were processed using the same overall procedures including concentration, trypsin digestion, SPE cleanup, and PRISM-SRM. Three technical replicates (three LC-SRM analyses) were also performed for each individually processed sample. The observed CV across the five process replicates was 6.6% for the transition 407.7/351.2 and 5.0% for the transition 407.7/476.3 (Table S3 and Supplemental Methods), illustrating good precision of the assay to measure low-abundance AGR2 in human urine.
LOQ of the assay was estimated based on S/N ≥10 and measurement CV <25%.27 Due to the presence of endogenous AGR2 in human urine and serum, LOQs were estimated using the samples with the lowest quantified AGR2 concentrations without spiking-in of recombinant AGR2 protein into human urine or serum matrix. Two selected cancer patient urine samples, P07-029CP and P06-003CP, have the lowest AGR2 concentrations at 3.0 pg and 12.1 pg per 100 μg total protein, respectively (Table 1). An average S/N of 6.7 was observed for P07-029CP, and 10.8 for P06-003CP, which suggested that LOQ for PRISM-SRM quantification of AGR2 in human urine is ~10 pg per 100 μg total urinary protein. To determine LOQ for human serum, three patient serum samples with lower AGR2 concentrations (130.9 pg/mL; 139.9 pg/mL; 111.7 pg/mL) were selected (Table S4), and their average S/N ratios were 9.7, 13, and 6.1, respectively. Thus, the LOQ of PRISM-SRM for quantification of AGR2 in human serum is ~130 pg/mL when combined with IgY14 immunoaffinity depletion.
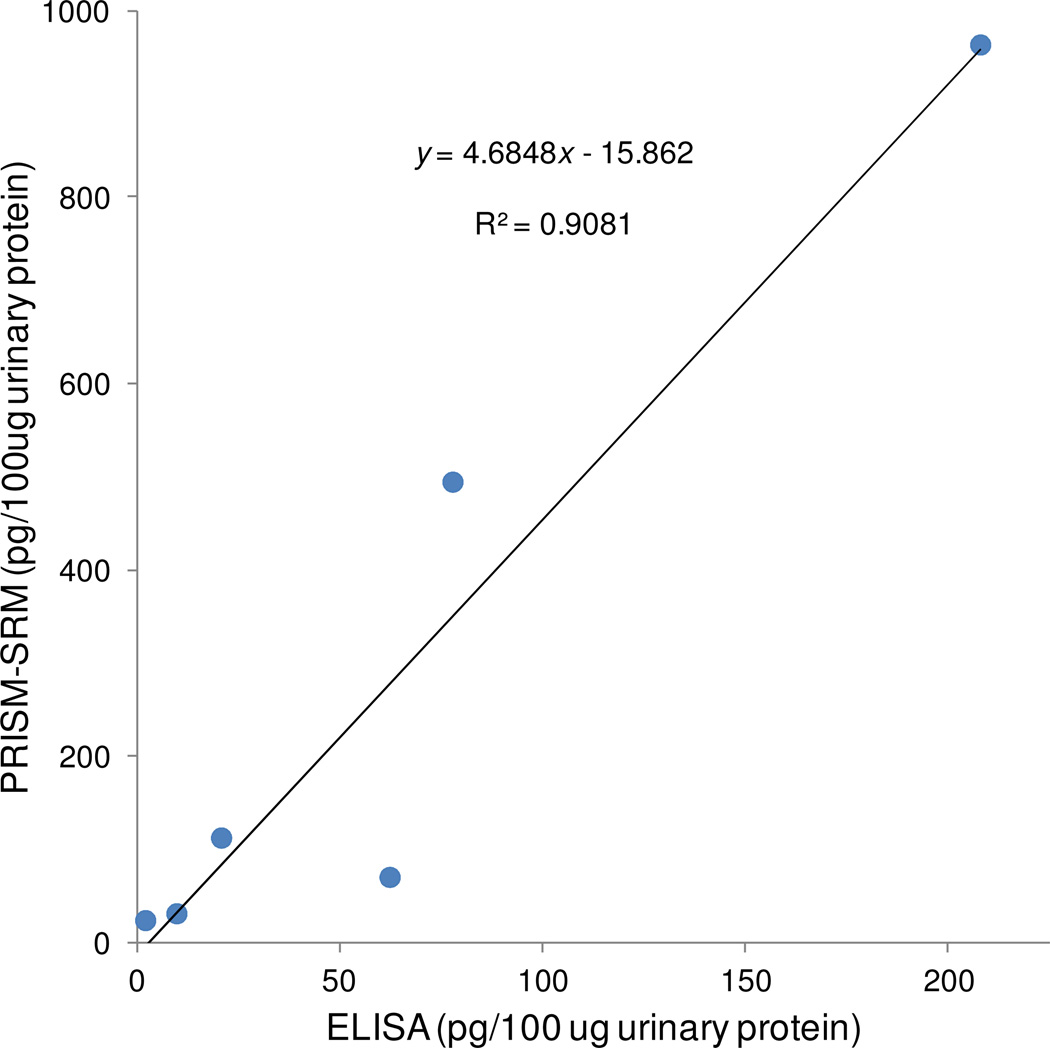
Correlation between PRISM-SRM and ELISA Results
To further confirm the accuracy of PRISM-SRM measurements, results for prostate patient urine samples measured by PRISM-SRM and ELISA were compared. In both assays, the concentrations were expressed as pg of AGR2 per 100 μg total urinary protein. A good correlation, R2 = 0.91, was observed (Figure 4). However, the AGR2 concentration measured by SRM was nearly 4.5-fold higher than that by ELISA.
Figure 4.
Correlation between PRISM-SRM and ELISA measurements of AGR2 in urine samples.
Ratio of Urinary AGR2/PSA Concentration as a Potential Biomarker
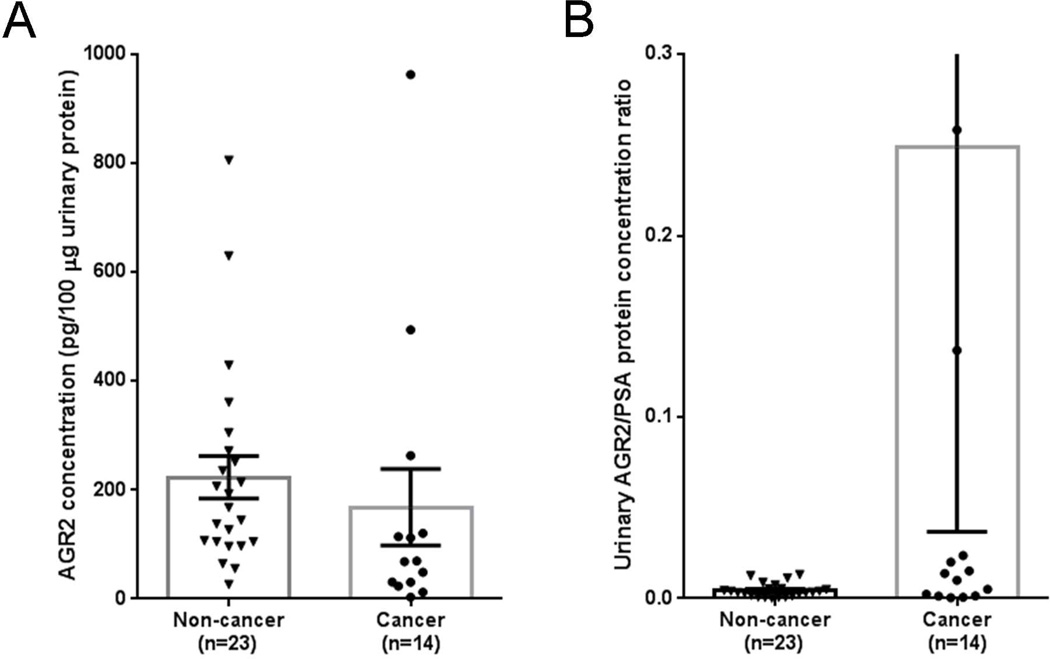
Urinary AGR2 concentrations between cancer (n = 14) and non-cancer (n = 23) subjects were compared; however, there was no significant difference (Kolmogorov-Smirnov test, P = 0.068) of AGR2 abundance between cancer (mean value: 168.2 pg/100 μg urinary protein) and non-cancer (median value: 223.4 pg/100 μg urinary protein) subjects (Figure 5A). Recently, in a clinical-grade PCA3 assay, a PCA3 score was generated by normalization of the expression levels of PCA3 mRNA to those of PSA mRNA (which served as a monitor for the abundance of prostate cancer cells).33 This concept of normalization in the PCA3 assay was adopted to calculate the ratio of urinary AGR2/PSA protein concentrations for all 37 samples. The L/H ratio of PSA in urine was obtained by direct LC-SRM measurements without PRISM due to the relatively high-abundance of PSA in male urine (Table S6). The urinary AGR2/PSA protein concentration ratio was calculated by dividing the L/H peak area ratio of AGR2 against the L/H peak area ratio of PSA because heavy internal standards were spiked into all urine samples at the same concentrations (Table S6). A significant difference (Kolmogorov-Smirnov test, P = 0.026) was observed for the urinary AGR2/PSA protein concentration ratios between cancer (mean value: 0.2488) and non-cancer (mean value: 0.0045) subjects (Figure 5B). These results suggested a potential utility of urinary AGR2/PSA ratio as a prostate cancer biomarker.
Figure 5.
AGR2 as a potential urine biomarker for prostate cancer. (A) AGR2 concentrations between non-cancer and cancer subjects (Kolmogorov-Smirnov test, P = 0.068); (B) AGR2/PSA concentration ratios between non-cancer and cancer subjects (Kolmogorov-Smirnov test, P = 0.026).
DISCUSSION
Since AGR2 is a secreted cancer-associated protein for many types of epithelial cancer, a sensitive and robust assay is needed for assessment of its utility in cancer diagnosis or prognosis. We described here the development of a sensitive PRISM-SRM assay for quantification of AGR2 at pg/mL levels in human urine and serum samples with high precision. While a relatively good correlation was observed between PRISM-SRM and ELISA measurements, the higher levels of AGR2 quantified by PRISM-SRM may be attributable to the detection mechanisms.27 Targeted mass spectrometry-based assays measure the total AGR2 in bodily fluids under denaturation conditions whereas ELISA measures AGR2 depending on antibody binding efficiency and epitope availability. Similar observations have been previously reported for SRM and ELISA measurement of total PSA in serum.34,35
Compared with antibody-based immunoassays, PRISM-SRM offers, in addition, multiplexing capability for simultaneously quantifying many proteins in complex biological samples.27 However, its overall throughput is reduced by the need of ~2 hour for capillary LC enrichment of target analytes. Unlike antibody-based immunoassays (where 96 or 384 samples can be processed concurrently), PRISM-SRM analyzes one sample at a time. Therefore, PRISM-SRM serves as a complementary and/or alternative technique to antibody-based immunoassays especially when high-quality antibodies for sensitive immunoassays are not available. Importantly, it could move otherwise intractable candidate protein markers into large-scale clinical validation studies.
In an initial cohort of urine samples, the AGR2 concentrations measured by PRISM-SRM ranged from 3.0 to 963 pg/100 μg total urinary protein mass. However, there is no significant difference in AGR2 concentrations between cancer and non-cancer subjects. These results seem to be contradictory to previous studies showing AGR2 mRNA expression highly elevated in prostate cancer patients.9,36 This discrepancy can be largely attributed to the large degree of variations in urinary protein concentration between subjects or within subjects, and therefore additional normalization procedures may be helpful for reducing sample variation. For example, the urinary PCA3 and TMPRSS2:ERG assays were both based on the normalization of PCA3 or TMPRSS2:ERG values with PSA mRNA.6,33 Similarly, urinary AGR2 mRNA assay was also normalized to PSA transcript in a report by Bu et al.,8 which demonstrated that the urinary AGR2/PSA mRNA ratio provided much better discrimination between cancer and benign than serum total PSA or % free PSA. The normalization strategy similar to AGR2 over PSA at the mRNA level has been adopted to normalize AGR2 protein concentration against urine PSA protein concentration as a measure of AGR2 contributed by the prostate. In cancer cases, an extra source of AGR2 is the tumor component in the prostate. Indeed, the urinary PSA by itself has no value in distinguish between cancer vs. non-cancer; however, the urinary AGR2/PSA ratio provided much better differentiation of cancer from non-cancer, and is potentially useful as a urine marker for prostate cancer. Additional evidence supporting the association of AGR2 with prostate cancer was the observed good correlation between serum AGR2 and PSA concentrations in the prostate cancer patient sera analyzed. This observation supports the hypothesis that both PSA and AGR2 are being released from the prostate.36 It was recently reported that AGR2 levels varied independently of PSA levels in prostate cancer.37 To the best of our knowledge, that data was generated by utilizing a not well-tried, newly developed commercial AGR2 ELISA assay to quantify serum AGR2 with measured levels of 10–2,500 ng/mL,37 which is much higher than that detected by our MS-based assay. Certain commercially available (polyclonal) antibodies are known to be notoriously nonspecific and cross-react with other proteins. Another indication of non-specificity was their detection of AGR2 in neuroendocrine tumors.38 These prostate tumors are negative for AGR2 expression. Without a rigorous characterization of the specificity of these ELISA reagents, the data generated can be problematic, especially when a thousand fold difference (pg/mL vs. ng/mL) in the quantification is observed when compared to our PRISM-SRM measurements. If AGR2 were in the ng/mL range (as PSA), it would have been more than likely discovered earlier by global proteomics profiling. Transcriptome data from sorted prostate cancer cells showed AGR2 array signal value was at least 3-fold lower than that of PSA.9 Our results confirmed the low-abundance levels of AGR2 in both urine and serum, and demonstrated that the robust PRISM-SRM assays provided superior sensitivity and specificity.
In conclusion, we have developed a highly sensitive PRISM-SRM assay for quantification of AGR2 at low pg/mL levels in prostate cancer patient urine and serum with high specificity. Our results indicate that urinary AGR2/PSA ratio is potentially useful for the differentiation of prostate cancer from non-cancer subjects. This assay should facilitate validation of AGR2 as a urine or serum biomarker for prostate cancer or other cancers using larger clinical cohorts.
Supplementary Material
ACKNOWLEDGMENTS
Portions of the research were supported by the NIH grant U01CA111244, NIH Director’s New Innovator Award Program DP2OD006668, NCI Early Detection Research Network Interagency Agreement Y01-CN-05013-29, U24-CA-16001901 from the National Cancer Institute Clinical Proteomic Tumor Analysis Consortium (CPTAC), and P41GM103493. The experimental work described herein was performed in the Environmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, a national scientific user facility sponsored by the DOE under Contract DE-AC05-76RL0 1830.
Abbreviations
- AGR2
anterior gradient 2
- CV
coefficient of variation
- ELISA
enzyme-linked immunosorbent assays
- XIC
extracted ion chromatogram
- LC
liquid chromatography
- L/H
peak area ratio of light to heavy peptides
- PSA
prostate-specific antigen
- S/N
signal-to-noise ratio
- SRM
selected reaction monitoring
- TFA
trifluoroacetic acid
Footnotes
ASSOCIATED CONTENT
Supporting Information Available
Supplemental tables and figures. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Siegel R, Naishadham D, Jemal A. CA Cancer J Clin. 2012;62:10. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Prakash A, Tomazela DM, Frewen B, Maclean B, Merrihew G, Peterman S, Maccoss M. J. Journal of proteome research. 2009;8:2733. doi: 10.1021/pr801028b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Placer J, Morote J. Arch Esp Urol. 2011;64:659. [PubMed] [Google Scholar]
- 4.Tomlins SA. Eur Urol. 2012 [Google Scholar]
- 5.Vlaeminck-Guillem V, Ruffion A, Andre J. Prog Urol. 2008;18:259. doi: 10.1016/j.purol.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 6.Tomlins SA, Aubin SM, Siddiqui J, Lonigro RJ, Sefton-Miller L, Miick S, Williamsen S, Hodge P, Meinke J, Blase A, Penabella Y, Day JR, Varambally R, Han B, Wood D, Wang L, Sanda MG, Rubin MA, Rhodes DR, Hollenbeck B, Sakamoto K, Silberstein JL, Fradet Y, Amberson JB, Meyers S, Palanisamy N, Rittenhouse H, Wei JT, Groskopf J, Chinnaiyan AM. Sci Transl Med. 2011;3:94ra72. doi: 10.1126/scitranslmed.3001970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maresh EL, Mah V, Alavi M, Horvath S, Bagryanova L, Liebeskind ES, Knutzen LA, Zhou Y, Chia D, Liu AY, Goodglick L. BMC Cancer. 2010;10:680. doi: 10.1186/1471-2407-10-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bu H, Bormann S, Schafer G, Horninger W, Massoner P, Neeb A, Lakshmanan VK, Maddalo D, Nestl A, Sultmann H, Cato AC, Klocker H. Prostate. 2011;71:575. doi: 10.1002/pros.21273. [DOI] [PubMed] [Google Scholar]
- 9.Wayner EA, Quek SI, Ahmad R, Ho ME, Loprieno MA, Zhou Y, Ellis WJ, True LD, Liu AY. Prostate. 2012;72:1023. doi: 10.1002/pros.21508. [DOI] [PubMed] [Google Scholar]
- 10.Chevet E, Fessart D, Delom F, Mulot A, Vojtesek B, Hrstka R, Murray E, Gray T, Hupp T. Oncogene. 2012 doi: 10.1038/onc.2012.346. [DOI] [PubMed] [Google Scholar]
- 11.Brychtova V, Vojtesek B, Hrstka R. Cancer Lett. 2011;304:1. doi: 10.1016/j.canlet.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Barraclough DL, Platt-Higgins A, de Silva Rudland S, Barraclough R, Winstanley J, West CR, Rudland PS. Am J Pathol. 2009;175:1848. doi: 10.2353/ajpath.2009.090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hrstka R, Nenutil R, Fourtouna A, Maslon MM, Naughton C, Langdon S, Murray E, Larionov A, Petrakova K, Muller P, Dixon MJ, Hupp TR, Vojtesek B. Oncogene. 2010;29:4838. doi: 10.1038/onc.2010.228. [DOI] [PubMed] [Google Scholar]
- 14.Pizzi M, Fassan M, Balistreri M, Galligioni A, Rea F, Rugge M. Appl Immunohistochem Mol Morphol. 2012;20:31. doi: 10.1097/PAI.0b013e3182233f9f. [DOI] [PubMed] [Google Scholar]
- 15.Fritzsche FR, Dahl E, Dankof A, Burkhardt M, Pahl S, Petersen I, Dietel M, Kristiansen G. Histol Histopathol. 2007;22:703. doi: 10.14670/HH-22.703. [DOI] [PubMed] [Google Scholar]
- 16.Valladares-Ayerbes M, Blanco-Calvo M, Reboredo M, Lorenzo-Patino MJ, Iglesias-Diaz P, Haz M, Diaz-Prado S, Medina V, Santamarina I, Pertega S, Figueroa A, Anton-Aparicio LM. Int J Mol Sci. 2012;13:4367. doi: 10.3390/ijms13044367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rice GE, Edgell TA, Autelitano DJ. J Exp Clin Cancer Res. 2010;29:62. doi: 10.1186/1756-9966-29-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edgell TA, Barraclough DL, Rajic A, Dhulia J, Lewis KJ, Armes JE, Barraclough R, Rudland PS, Rice GE, Autelitano DJ. Clin Sci (Lond) 2010;118:717. doi: 10.1042/CS20090537. [DOI] [PubMed] [Google Scholar]
- 19.Vivekanandan P, Micchelli ST, Torbenson M. Hum Pathol. 2009;40:293. doi: 10.1016/j.humpath.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen R, Pan S, Duan X, Nelson BH, Sahota RA, de Rham S, Kozarek RA, McIntosh M, Brentnall TA. Mol Cancer. 2010;9:149. doi: 10.1186/1476-4598-9-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dumartin L, Whiteman HJ, Weeks ME, Hariharan D, Dmitrovic B, Iacobuzio-Donahue CA, Brentnall TA, Bronner MP, Feakins RM, Timms JF, Brennan C, Lemoine NR, Crnogorac-Jurcevic T. Cancer Res. 2011;71:7091. doi: 10.1158/0008-5472.CAN-11-1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi T, Su D, Liu T, Tang K, Camp DG, 2nd, Qian WJ, Smith RD. Proteomics. 2012;12:1074. doi: 10.1002/pmic.201100436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Picotti P, Aebersold R. Nat Meth. 2012;9:555. doi: 10.1038/nmeth.2015. [DOI] [PubMed] [Google Scholar]
- 24.Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA. Mol Cell Proteomics. 2007;6:2212. doi: 10.1074/mcp.M700354-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoofnagle AN, Becker JO, Wener MH, Heinecke JW. Clin Chem. 2008;54:1796. doi: 10.1373/clinchem.2008.109652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whiteaker JR, Zhao L, Abbatiello SE, Burgess M, Kuhn E, Lin C, Pope ME, Razavi M, Anderson NL, Pearson TW, Carr SA, Paulovich AG. Mol Cell Proteomics. 2011;10 doi: 10.1074/mcp.M110.005645. M110 005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi T, Fillmore TL, Sun X, Zhao R, Schepmoes AA, Hossain M, Xie F, Wu S, Kim JS, Jones N, Moore RJ, Pasa-Tolic L, Kagan J, Rodland KD, Liu T, Tang K, Camp DG, 2nd, Smith RD, Qian W. J. Proc Natl Acad Sci U S A. 2012;109:15395. doi: 10.1073/pnas.1204366109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.True LD, Zhang H, Ye M, Huang CY, Nelson PS, von Haller PD, Tjoelker LW, Kim JS, Qian WJ, Smith RD, Ellis WJ, Liebeskind ES, Liu AY. Mod Pathol. 2010 doi: 10.1038/modpathol.2010.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qian WJ, Kaleta DT, Petritis BO, Jiang H, Liu T, Zhang X, Mottaz HM, Varnum SM, Camp DG, 2nd, Huang L, Fang X, Zhang WW, Smith RD. Mol Cell Proteomics. 2008;7:1963. doi: 10.1074/mcp.M800008-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fusaro VA, Mani DR, Mesirov JP, Carr SA. Nat Biotechnol. 2009;27:190. doi: 10.1038/nbt.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eyers CE, Lawless C, Wedge DC, Lau KW, Gaskell SJ, Hubbard S. J. Molecular & Cellular Proteomics. 2011;10 doi: 10.1074/mcp.M110.003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss M. J. Bioinformatics. 2010;26:966. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marks LS, Bostwick DG. Rev Urol. 2008;10:175. [PMC free article] [PubMed] [Google Scholar]
- 34.Liu T, Hossain M, Schepmoes AA, Fillmore TL, Sokoll LJ, Kronewitter SR, Izmirlian G, Shi T, Qian W-J, Leach RJ, Tompson IM, Chan DW, Smith RD, Kagan J, Srivastava S, Rodland KD, Camp DG. J Proteomics. 2012 doi: 10.1016/j.jprot.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi T, Sun X, Gao Y, Fillmore TL, Schepmoes AA, Zhao R, He J, Moore RJ, Kagan J, Rodland KD, Liu T, Liu AY, Smith RD, Tang K, Camp DG, 2nd, Qian WJ. J Proteome Res. 2013;12:3353. doi: 10.1021/pr400178v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang JS, Gong A, Cheville JC, Smith DI, Young CY. Genes Chromosomes Cancer. 2005;43:249. doi: 10.1002/gcc.20188. [DOI] [PubMed] [Google Scholar]
- 37.Kani K, Malihi PD, Jiang Y, Wang H, Wang Y, Ruderman DL, Agus DB, Mallick P, Gross ME. Prostate. 2012 doi: 10.1002/pros.22569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.