Abstract
Here, we present 2 case reports of patients with desmoplastic small round cell tumor (DSRCT), a very rare and aggressive mesenchymal cancer, and we discuss 2therapeutic options for this sarcoma. This report focuses on men aged 22 and 37 years, respectively. The first patient presented with an abdominopelvic mass which was not suitable for surgery. He underwent chemotherapy (adriblastina and cisplatin) with a brief partial remission and survival time of 13 months. The second patient presented with an abdominal mass and underwent partial resection. He received chemotherapy and bevacizumab, resulting in a partial remission and a survival time of 34 months. The extent of surgery and monoclonal antibody use probably had a positive impact on survival. It is necessary to include specific targeted therapies in an attempt to improve survival. Surprisingly, positron emission tomography was not effective in restaging of the second patient, emphasizing the importance of computed tomography or magnetic resonance in DSRCT.
Key words: Desmoplastic small round cell tumor, Chemotherapy, Abdominal tumor, Bevacizumab
Introduction
Desmoplastic small round cell tumor (DSRCT) is a very rare and aggressive mesenchymal cancer, characterized by intraperitoneal masses without an apparent organ of origin [1]. Metastases are most common to the liver and lungs [2]. It resembles other small round cell tumors [3] as described by Gerald and Rosai in 1989 [4]. There are fewer than 200 reported cases [2]. It occurs mainly in children, young adults, and males [5, 6] with an average age of 22 years [7].
Histologically, it is characterized by clustering of small round cells separated by desmoplastic stroma [2]. In immunohistochemistry (IH) there are epithelial, mesenchymal, and positive neuronal markers [8]. The translocation t(11; 22)(p13; q12) is considered exclusive to DSRCT [2]. There is no therapeutic pattern for DSRCT [1]. Chemotherapy and radiotherapy cause temporary improvement and are therefore essential for surgical cytoreduction for increasing survival time.
Case Reports
Case 1
A 22-year-old male presented with a 3-month history of intensive backaches and weight loss. Later, he noticed a mass in the hypogastrium region and lower limb lymphedema. It evolved into intestinal semiocclusion.
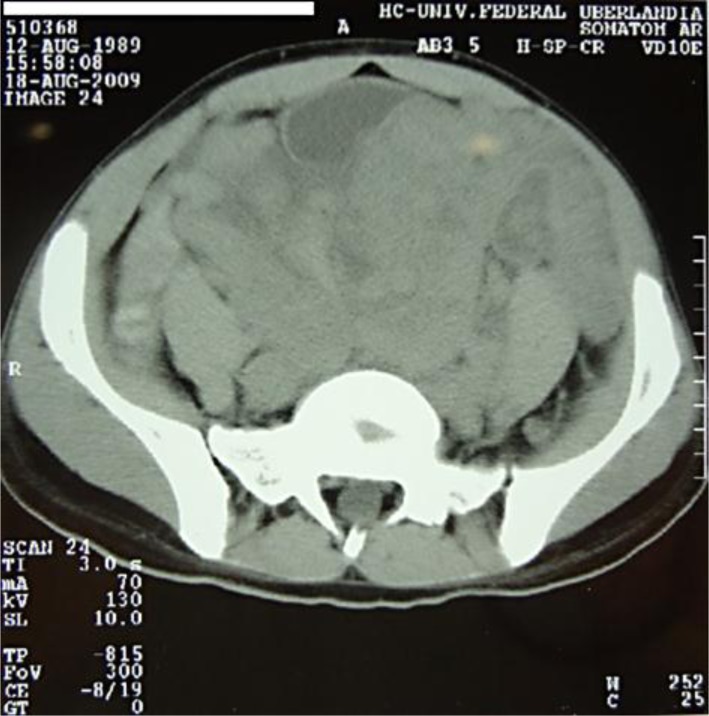
On computed tomography (CT), performed on August 18, 2009, the mass was confirmed to be a retrovesical tumor (fig. 1), extending to the umbilical region, with peritoneal implants and the liver capsule (7.0 cm). A laparoscopy with biopsy was done, confirming an unresectable retroperitoneal mass. Histology and IH studies (table 1) confirmed the diagnosis of DSRCT.
Fig. 1.
Case 1: CT scan showing a retrovesical tumor.
Table 1.
IH studies compatible with DSRCT
| Antibody used | Case 1 | Case 2 |
|---|---|---|
| Anti-cytokeratins | Positive | Positive |
| Anti-EMA/E29 | Positive | – |
| Anti-desmin/D33 | Positive | Positive |
| Anti-NSE | Positive | – |
| Anti-vimentin | Positive | – |
| Anti-gene MIC2 (CD99) | Positive | Negative |
| Anti-chromogranin | Negative | Negative |
| Anti-LCA | Negative | – |
| Anti-protein S100 | Focal positivity | – |
| Anti-synaptophysin | Negative | Negative |
| Cytokeratins 8 and 18 | – | Positive |
| Enolase | – | Positive |
| Cytokeratin 20 | – | Negative |
| CDX-2 | – | Negative |
On September 19, 2009, with a performance status (PS) of 3, the patient began chemotherapy [doxorubicin 60 mg/m2 and cisplatin 60 mg/m2 on day (D)1], as available from the public service. On D15, resolution of the bowel obstruction was noted. After the 3rd cycle of chemotherapy, abdominal CT still showed a solid heterogeneous mass, but with a reduction of 42%. After the 8th cycle of chemotherapy, on May 3, 2010, a new CT scan showed stabilization of the lesions; however, they remained inoperable. The patient was referred for outpatient palliative care. On September 30, 2010, he unfortunately progressed to death due to neoplastic cachexia. Since the initial diagnosis, he had had a survival time of 13 months.
Case 2
A 37-year-old male presented with a 2-month history of poor digestion and weight loss of 7 kg. On clinical examination, a right abdominal tumor mass was found. Magnetic resonance imaging (MRI) confirmed an abdominal lesion, measuring 18.9 × 16.6 cm, on the periphery of the liver segment VI. A chest CT scan was normal. At exploratory laparotomy, on October 7, 2008, a tumor attached to the liver, invading the splenic hilum, omentum, and mesenteric region was found. Partial resection was possible. Histology and IH studies (table 1) were compatible with DSRCT.
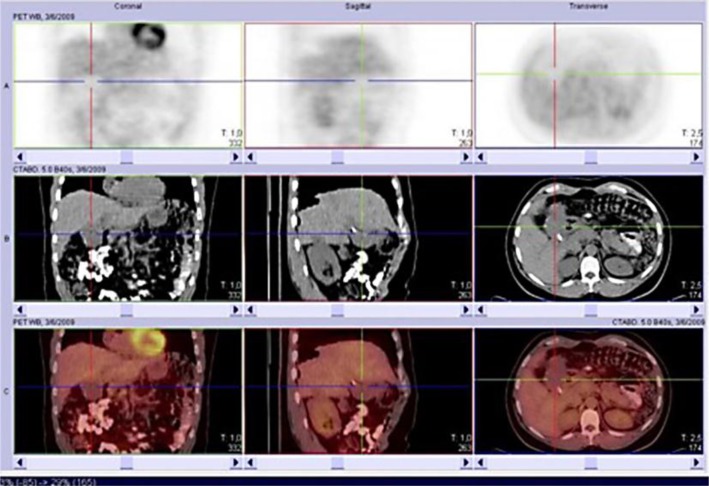
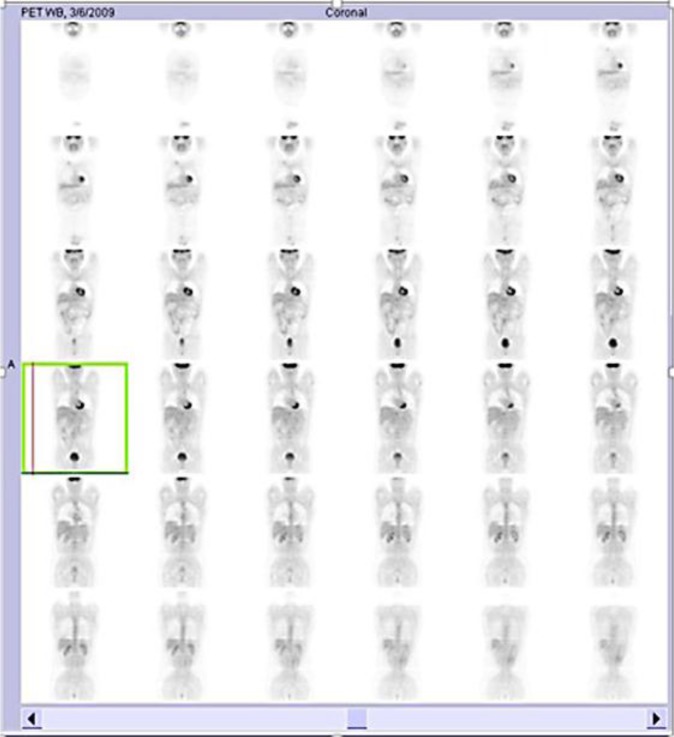
He started chemotherapy (carboplatin AUC5, paclitaxel 180 mg/m2, and bevacizumab 7.5 mg/kg) on November 18, 2008, for 21/21 days, with a covenant provided by private health insurance. He had PS 3 (73 kg) and changes in liver function. After the 3rd round of chemotherapy, on January 16, 2009, MRI already showed partial remission (78.6% reduction). After the 8th cycle, on May 12, 2009, a presurgical positron emission tomography (PET) reassessment was performed. Interestingly, PET showed physiological distribution of F-18-fluorodeoxyglucose (FDG) without identifying any uptake (fig. 2, fig. 3). A concomitant CT scan showed tumor infiltration of the hilum, hepatic parenchyma, and peritoneal implants (fig. 2).
Fig. 2.
Case 2: CT scans showing hilum and hepatic parenchyma infiltration (PET without any uptake).
Fig. 3.
Case 3: physiological distribution of FDG shown on PET without any uptake.
A new surgical approach was contraindicated. On June 16, 2009, chemotherapy was stopped at the patient's request. Two months after that, there was a clinical and liver function worsening due to tumor progression and compression of the biliary tract. A retrograde endoscopic cholangiopancreatography was done and a stent was successfully placed.
Chemotherapy was restarted on August 26, 2009. He already had new partial remission. This scheme was discontinued after the 10th cycle on March 30, 2010 (4.9-cm tumor) due to more frequent occurrences of neutropenia. Chemotherapy was then initiated with cyclophosphamide 50 mg/day, oral, and bevacizumab; this regimen was maintained until November 16, 2010, when it was suspended because of worsening of the patient's clinical state and laboratory findings. He remained in hospice care until his death on July 22, 2011, due to neoplastic cachexia and hepatic encephalopathy. He had had a survival time of 34 months after the initial diagnosis.
Discussion
We presented 2 cases in men, 22 and 37 years old, respectively, emphasizing that DSRCT occurs predominantly in young males [2, 7, 9]. There is no known ethnic predisposition [1]. The common symptoms are abdominal distention or pain, palpable mass, and weight loss [8] which intensifies with tumoral progression [2, 10]. Due to unspecific complaints, the diagnosis is always late, compromising the curative treatment, as in the 2 cases presented here, with advanced abdominal disease [7, 9]. Hepatic metastases are found in 42% of cases, less frequently in the lungs [1] and rarely in the bones or bone marrow [10].
There are no known risks or etiological factors [1]. DSRCT presents an exclusive translocation, t(11,22)(p13;q12), resulting in EWS and WTI gene fusion [1, 7, 8, 9, 10, 11, 12, 13, 14], which occurs mainly in the embryonic mesenchymal cells of the serosa of the abdominal organs [7]. The primary tumor's organ of origin could not be detected in our patients, nor did we do a genetic study since there was conclusive IH, with epithelial, mesenchymal, and some positive neuronal markers [1, 2, 7, 8, 9, 15]. Histology also confirmed the small rounded cell pattern [9]. When histology is inconclusive, in up to 50% of cases, IH or genetics might help [10].
CT is the most used imaging exam [1], though it might produce false-positive results [9]. In case 1, CT was used in the response evaluation and progression. In case 2, both CT and MRI were efficient with respect to diagnosis and follow-up, but PET did not show the tumor with physiological distribution of FDG. New cases are required to confirm this possible characteristic of DSRCT.
Wide tumoral resection is the prognostic factor that impacts the most on an increased survival rate [2], with an average of 34 months’ survival time. In an inoperable case, survival time is 14 months on average. Our cases confirm the data: case 1 only underwent biopsy and survived 13 months; case 2 had a larger cytoreduction and survived 34 months. A longer survival time occurs in 15–30% of cases [3], and up to 18% will be alive in 5 years after undergoing wide surgical resection [9, 13].
Due to DSRCT's rarity, there is no standard treatment procedure. The average survival time with polychemotherapy is 32 months [13], with relapse being the rule [3]. In well-selected cases, intraperitoneal chemotherapy might be used after surgery with minimal residual disease [2]. The P6 protocol seems to increase the survival rate [12], alternating cyclophosphamide, doxorubicin, and vincristine with ifosfamide and etoposide, although it is highly myelotoxic. Case 2, treated with bevacizumab associated with chemotherapy, showed a longer partial remission; however, he had a more extensive surgery.
Conclusion
The 2 reports were of men aged 22 and 37 years, respectively. The first patient presented with an abdominopelvic mass which was not suitable for surgery. He underwent chemotherapy (adriblastina and cisplatin) with a brief partial remission and survival time of 13 months. The second patient had an abdominal mass which was partially resected. He received chemotherapy (paclitaxel and carboplatin) and bevacizumab, also with partial remission, but with a survival time of 34 months. PET was not effective in restaging of the second patient, emphasizing the importance of CT or MRI in DSRCT.
Most patients have nonspecific symptoms, delaying diagnosis, and worsening the prognosis, since there are few effective treatment protocols. It is necessary to include specific targeted therapies in an attempt to improve survival.
Disclosure Statement
No research support was received for the writing of this paper.
References
- 1.Koniari K, Mahera H, Nikolaou M, et al. Intraabdominal desmoplastic small round cell tumor: report of a case and literature review. Int J Surg Case Rep. 2011;2:293–296. doi: 10.1016/j.ijscr.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dufresne A, Cassier P, Couraud L, et al. Desmoplastic small round cell tumor: current management and recent findings. Sarcoma. 2012;2012:714986. doi: 10.1155/2012/714986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perna MJ, Streck CJ: A large solitary desmoplastic small round cell tumor. Annu Sci Meet, Southeastern Surg Congr, Birmingham, AL, 2012. [PubMed]
- 4.Gerald WL, Rosai J. Case 2. Desmoplastic small cell tumor with divergent differentiation. Pediatr Pathol. 1989;9:177–183. doi: 10.3109/15513818909022347. [DOI] [PubMed] [Google Scholar]
- 5.Órdoñez NG, El-Naggar AK, Ro JY, et al. Intra-abdominal desmoplastic small cell tumor: a light microscopic, immunocytochemical, ultrastructural, and flow cytometric study. Hum Pathol. 1993;24:850–865. doi: 10.1016/0046-8177(93)90135-4. [DOI] [PubMed] [Google Scholar]
- 6.Kretschmar CS, Colbach C, Bhan I, et al. Desmoplastic small cell tumor: a report of three cases and a review of the literature. J Pediat Hematol/Oncol. 1996;18:293–298. doi: 10.1097/00043426-199608000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Gerald WL, Ladanyi M, Alava E, et al. Clinical, pathologic, and molecular spectrum of tumors associated with t(11;22)(p13;q12): desmoplastic small round-cell tumor and its variants. J Clin Oncol. 1998;16:3028–3036. doi: 10.1200/JCO.1998.16.9.3028. [DOI] [PubMed] [Google Scholar]
- 8.Ogata DC, Tatsugui JT, Machuca TN, et al. Desmoplastic round small cell tumor: a case report of a neoplasm of difficult diagnosis. Rev Bras Cancer. 2005;51:263–266. [Google Scholar]
- 9.Cao L, Ni J, Que R, et al. Desmoplastic small round cell tumor: a clinical, pathological, and immunohistochemical study of 18 Chinese cases. Int J Surg Pathol. 2008;16:257–262. doi: 10.1177/1066896907306124. [DOI] [PubMed] [Google Scholar]
- 10.Philippe-Chomette P, Kabbara N, Andre N, et al. Desmoplastic small round cell tumors with EWS-WT1 fusion transcript in children and young adults. Pediat Blood Cancer. 2012;58:891–897. doi: 10.1002/pbc.23403. [DOI] [PubMed] [Google Scholar]
- 11.Torres US, Ribeiro MCA, Souza AS, et al. Abdominal desmoplastic small round cell tumor of childhood: case report. J Bras Patol Med Lab. 2010;46:55–59. [Google Scholar]
- 12.Kushner BH, LaQuaglia MP, Wollner N, et al. Desmoplastic small round-cell tumor: prolonged progression-free survival with aggressive multimodality therapy. J Clin Oncol. 1996;14:1526–1531. doi: 10.1200/JCO.1996.14.5.1526. [DOI] [PubMed] [Google Scholar]
- 13.Gil A, Portilla AG, Brun EA, et al. Clinical perspective on desmoplastic small round-cell tumor. Oncology. 2004;67:231–242. doi: 10.1159/000081323. [DOI] [PubMed] [Google Scholar]
- 14.Mrabti H, Kaikani W, Ahbeddou N, et al. Metastatic desmoplastic small round cell tumor controlled by an anthracycline-based regimen: review of the role of chemotherapy. J Gastrointest Cancer. 2012;43:103–109. doi: 10.1007/s12029-011-9260-6. [DOI] [PubMed] [Google Scholar]
- 15.Westphalen ACA, Ferreira JHP, Daudt AW, et al. Intaadbominal desmoplastic small round cell tumor: case report (in Portuguese) Radiol Bras. 2001;34:299–304. [Google Scholar]