Abstract
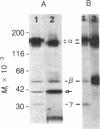
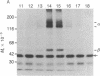
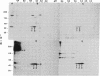
Physiological studies indicate that voltage-sensitive calcium channels are regulated by cAMP and protein phosphorylation. The calcium antagonist receptor of the voltage-sensitive calcium channel from transverse-tubule membranes consists of three subunits, designated alpha, beta, and gamma. The catalytic subunit of cAMP-dependent protein kinase phosphorylates both the alpha and beta subunits of the purified receptor at a rate and extent that suggests they are potential physiological substrates of this enzyme. The phosphorylation of the alpha and beta subunits in transverse-tubule membranes was analyzed by two-dimensional gel electrophoresis. In intact transverse-tubule membranes, the alpha subunit is not significantly phosphorylated. However, the beta subunit, identified by its Mr, pI, and binding to wheat germ agglutinin-Sepharose, was one of the substrates selectively phosphorylated by cAMP-dependent protein kinase in transverse-tubule membranes. These results suggest that cAMP-dependent phosphorylation of the beta subunit of the calcium antagonist receptor may be an important regulatory mechanism for calcium channel function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Jeboory A. A., Marshall R. J. Correlation between the effects of salbutamol on contractions and cyclic AMP content of isolated fast-and slow-contracting muscles of the guinea pig. Naunyn Schmiedebergs Arch Pharmacol. 1978 Dec;305(3):201–206. doi: 10.1007/BF00498811. [DOI] [PubMed] [Google Scholar]
- Almers W., Fink R., Palade P. T. Calcium depletion in frog muscle tubules: the decline of calcium current under maintained depolarization. J Physiol. 1981 Mar;312:177–207. doi: 10.1113/jphysiol.1981.sp013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtel P. J., Beavo J. A., Krebs E. G. Purification and characterization of catalytic subunit of skeletal muscle adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Apr 25;252(8):2691–2697. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brum G., Flockerzi V., Hofmann F., Osterrieder W., Trautwein W. Injection of catalytic subunit of cAMP-dependent protein kinase into isolated cardiac myocytes. Pflugers Arch. 1983 Jul;398(2):147–154. doi: 10.1007/BF00581064. [DOI] [PubMed] [Google Scholar]
- Caswell A. H., Baker S. P., Boyd H., Potter L. T., Garcia M. beta-adrenergic receptor and adenylate cyclase in transverse tubules of skeletal muscle. J Biol Chem. 1978 May 10;253(9):3049–3054. [PubMed] [Google Scholar]
- Catterall W. A., Morrow C. S., Hartshorne R. P. Neurotoxin binding to receptor sites associated with voltage-sensitive sodium channels in intact, lysed, and detergent-solubilized brain membranes. J Biol Chem. 1979 Nov 25;254(22):11379–11387. [PubMed] [Google Scholar]
- Chiarandini D. J., Stefani E. Calcium action potentials in rat fast-twitch and slow-twitch muscle fibres. J Physiol. 1983 Feb;335:29–40. doi: 10.1113/jphysiol.1983.sp014516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M. R., Casnellie J. E., Catterall W. A. Selective phosphorylation of the alpha subunit of the sodium channel by cAMP-dependent protein kinase. J Biol Chem. 1982 Jul 25;257(14):7918–7921. [PubMed] [Google Scholar]
- Costa M. R., Catterall W. A. Cyclic AMP-dependent phosphorylation of the alpha subunit of the sodium channel in synaptic nerve ending particles. J Biol Chem. 1984 Jul 10;259(13):8210–8218. [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochemistry. 1984 May 8;23(10):2113–2118. doi: 10.1021/bi00305a001. [DOI] [PubMed] [Google Scholar]
- Curtis B. M., Catterall W. A. Solubilization of the calcium antagonist receptor from rat brain. J Biol Chem. 1983 Jun 25;258(12):7280–7283. [PubMed] [Google Scholar]
- Doroshenko P. A., Kostyuk P. G., Martynyuk A. E. Intracellular metabolism of adenosine 3',5'-cyclic monophosphate and calcium inward current in perfused neurones of Helix pomatia. Neuroscience. 1982;7(9):2125–2134. doi: 10.1016/0306-4522(82)90124-5. [DOI] [PubMed] [Google Scholar]
- Doroshenko P. A., Kostyuk P. G., Martynyuk A. E., Kursky M. D., Vorobetz Z. D. Intracellular protein kinase and calcium inward currents in perfused neurones of the snail Helix pomatia. Neuroscience. 1984 Jan;11(1):263–267. doi: 10.1016/0306-4522(84)90229-x. [DOI] [PubMed] [Google Scholar]
- Fedulova S. A., Kostyuk P. G., Veselovsky N. S. Calcium channels in the somatic membrane of the rat dorsal root ganglion neurons, effect of cAMP. Brain Res. 1981 Jun 9;214(1):210–214. doi: 10.1016/0006-8993(81)90457-1. [DOI] [PubMed] [Google Scholar]
- Fosset M., Jaimovich E., Delpont E., Lazdunski M. [3H]nitrendipine receptors in skeletal muscle. J Biol Chem. 1983 May 25;258(10):6086–6092. [PubMed] [Google Scholar]
- Garrison J. C., Wagner J. D. Glucagon and the Ca2+-linked hormones angiotensin II, norepinephrine, and vasopressin stimulate the phosphorylation of distinct substrates in intact hepatocytes. J Biol Chem. 1982 Nov 10;257(21):13135–13143. [PubMed] [Google Scholar]
- Glossmann H., Ferry D. R., Boschek C. B. Purification of the putative calcium channel from skeletal muscle with the aid of [3H]-nimodipine binding. Naunyn Schmiedebergs Arch Pharmacol. 1983 Jun;323(1):1–11. doi: 10.1007/BF00498821. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Serratos H., Hill L., Valle-Aguilera R. Effects of catecholamines and cyclic amp on excitation--contraction coupling in isolated skeletal muscle fibres of the frog. J Physiol. 1981 Jun;315:267–282. doi: 10.1113/jphysiol.1981.sp013747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefrath S. T., Smith P. B., Appel S. H. Characterization of the beta-adrenergic receptor and adenylate cyclase in skeletal muscle plasma membranes. Arch Biochem Biophys. 1978 Jun;188(2):328–337. doi: 10.1016/s0003-9861(78)80017-4. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. The sodium channel from rat brain. Purification and subunit composition. J Biol Chem. 1984 Feb 10;259(3):1667–1675. [PubMed] [Google Scholar]
- Hofmann F., Bechtel P. J., Krebs E. G. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977 Feb 25;252(4):1441–1447. [PubMed] [Google Scholar]
- Horne P., Triggle D. J., Venter J. C. Nitrendipine and isoproterenol induce phosphorylation of a 42,000 dalton protein that co-migrates with the affinity labeled calcium channel regulatory subunit. Biochem Biophys Res Commun. 1984 Jun 29;121(3):890–898. doi: 10.1016/0006-291x(84)90761-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Osterrieder W., Brum G., Hescheler J., Trautwein W., Flockerzi V., Hofmann F. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature. 1982 Aug 5;298(5874):576–578. doi: 10.1038/298576a0. [DOI] [PubMed] [Google Scholar]
- Reuter H. Localization of beta adrenergic receptors, and effects of noradrenaline and cyclic nucleotides on action potentials, ionic currents and tension in mammalian cardiac muscle. J Physiol. 1974 Oct;242(2):429–451. doi: 10.1113/jphysiol.1974.sp010716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Scholz H. The regulation of the calcium conductance of cardiac muscle by adrenaline. J Physiol. 1977 Jan;264(1):49–62. doi: 10.1113/jphysiol.1977.sp011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautwein W., Taniguchi J., Noma A. The effect of intracellular cyclic nucleotides and calcium on the action potential and acetylcholine response of isolated cardiac cells. Pflugers Arch. 1982 Feb;392(4):307–314. doi: 10.1007/BF00581624. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Weingart R. Inotropic effect of cyclic AMP in calf ventricular muscle studied by a cut end method. J Physiol. 1976 Aug;260(1):117–141. doi: 10.1113/jphysiol.1976.sp011507. [DOI] [PMC free article] [PubMed] [Google Scholar]