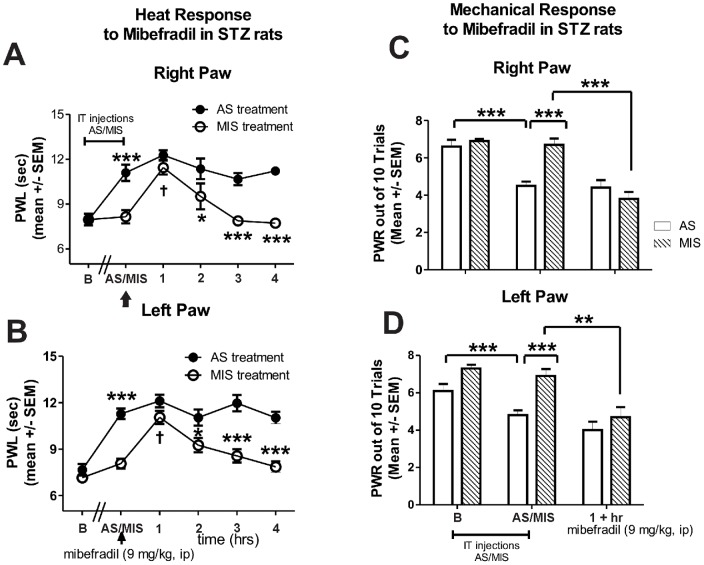
Figure 7. Knock-down of CaV3.2 abolishes mibefradil-induced alleviation of heat hyperalgesia and mechanical hypersensitivity in diabetic rats.
Cav3.2 T-channels in sensory neurons were knocked-down with intrathecal administration (every 12 hrs for a total of 4 doses) of oligodeoxynucleotide (AS) specific for the Cav3.2 T-channels. Controls were given mismatch oligodeoxynucleotide (MIS). Heat hyperalgesia was quantified with PWLs (A, right paw; B, left paw) and mechanical hypersensitivity was measured using PWRs (C, right paw; D, left paw). Compared with baseline recordings (marked as B), AS-treated diabetic rats exhibited a significant increase in PWLs (***, p<0.001) and a significant decrease in PWRs (***, p<0.001; marked as AS/MIS). At that point, mibefradil was administered (indicated with an arrow) and heat hyperalgesia was assessed hourly. Mibefradil (at 9 mg/kg i.p.) had no further effect to alleviate heat hyperalgesia; i.e., PWLs recordings remained stable (and significantly elevated compared with the MIS-treated STZ rats; *, p<0.05; ***, p<0.001) throughout the four-hour period post-mibefradil administration (closed circles). In MIS-treated diabetic rats, mibefradil induced a transient increase in PWLs (†, p<0.05 at 1 hr post-injection). Mechanical sensitivity assessment was initiated 1 h post mibefradil treatment when the effect was shown to be most significant (as indicated in Fig. 3C and D). PWRs in AS-treated diabetic rats post-mibefradil injection remain unchanged whereas in MIS-treated diabetic rats there was a significant effect of mibefradil marked by a decrease in PWRs (***, p<0.001, right paw; **, p<0.01, left paw; n = 5 rats per data point).