Figure 9. Mibefradil and Cav3.2 antisense are effective in alleviating diabetic cold allodynia.
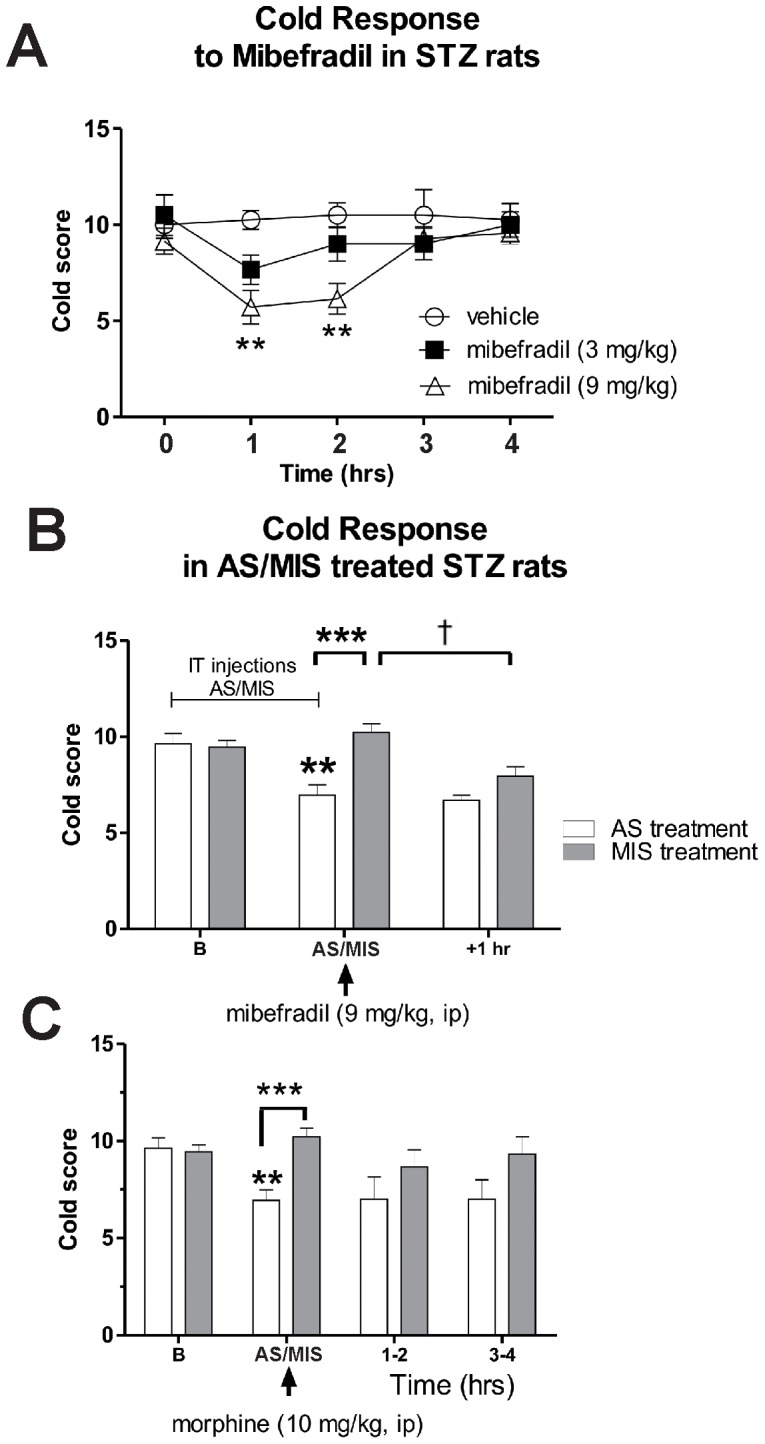
Cav3.2 T-channels in sensory neurons were knocked down with intrathecal administration (every 12 hrs for a total of 4 doses) of oligodeoxynucleotide (AS) specific for the Cav3.2 T-channels. Controls were given mismatch oligodeoxynucleotide (MIS). Cold allodynia was quantified using cold score (see Methods). A) Mibefradil induced dose-dependent alleviation of cold allodynia in diabetic rats compared with vehicle treatment. At 9 mg/kg, i.p., it caused approximately a 45% decrease in pain scores (**, p<0.01) compared with vehicle treatment, an effect that lasted a couple of hours. Although mibefradil, at 3 mg/kg, i.p., induced a slight decrease in cold scores, the effect was very transient and non-significant. B) AS-CaV3.2 treatment had significant antiallodynic effect compared with the baseline (†††, p<0.001) and MIS-treatment (***, p<0.001). The antiallodynic effect of mibefradil was blocked with AS-CaV3.2 (i.e., cold scores in AS-treated animals before and after mibefradil injection were not different), but not with MIS-CaV3.2 (i.e. there was a significant decrease in cold scores in MIS-treated animals after mibefradil treatment compared with cold scores before the treatment; †, p<0.01). C) Unlike mibefradil, morphine was completely ineffective in alleviating cold allodynia in both AS- and MIS-diabetic rats at any of the time points. (††, p<0.01 in AS-treated diabetic animals compared with the baseline; ***, p<0.001 compared with MIS-treated diabetic animals) (n = 3–11 rats per data point).
