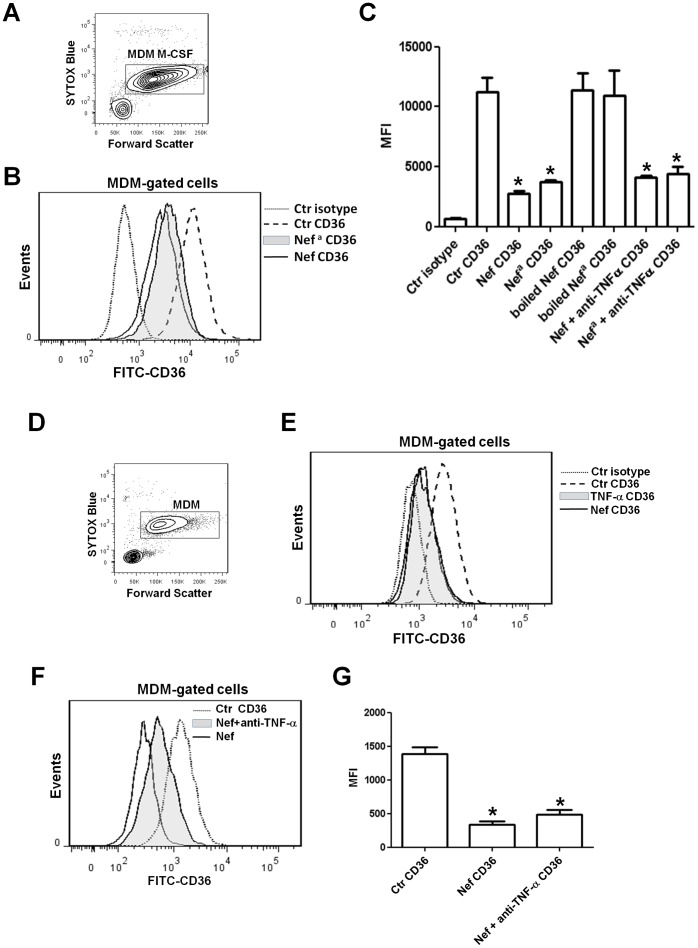
Figure 11. Nef-induced TNF-α release does not explain the downregulation of CD36 expression in MDMs.
Cells isolated by using CD14 magnetic beads (Miltenyi Biotec) were cultured in presence of human M-CSF (10 ng/mL) for 5 days and for additional three days in presence of two rNef/myr from different manufactures, the inactivated rNef/myr proteins by boiling or in presence of the rNef/myr proteins together with anti-human TNF-α antibody (1 μg/mL). (A) The dot plot shows the viability of cells by using SYTOX Blue dead cell stain. MDMs are identified by rectangular gate (MDM M-CSF) and analyzed for CD36 expression by using FITC-conjugated CD36 antibody. (B) The relative fluorescence intensities of CD36 in Nef-treated (solid line), Nefa-treated (solid grey histogram) and untreated (dash line) cells are shown in the representative histogram. Matched isotype (dotted line) was used as control of non-specific fluorescence signals (Nef refers to the protein from Dr. M. Federico [22]; Nefa, to the protein from Jena Bioscience). (C) The column bar graph presents the MFI of untreated cells (Ctr CD36), Nef- and Nefa-treated cells (Nef CD36 and Nefa CD36), cells incubated with inactivated Nef proteins (boiled Nef CD36 and boiled Nefa CD36) and cells incubated with the Nef proteins and anti-humanTNF-α (Nef+anti TNFα CD36) stained with FITC-conjugated anti-CD36. The results (mean ± standard deviation) are representative of three independent experiments (*p<0.05). (D) PBMCs were cultivated in HEMA condition w/o EPO for three days and for additional three days in presence of TNF-α (10 ng/mL) or rNef/myr as control of CD36 downregulation. The viability of cells, assessed by SYTOX Blue dead cell stain, is shown in the panel D. MDMs are identified by rectangular gate (MDM) and analyzed for CD36 expression by using FITC-conjugated CD36 antibody. (E) The relative fluorescence intensities of CD36 in Nef-treated (solid line), TNF-α-treated (solid grey histogram) and untreated (dash line) cells are shown in the representative histogram. Matched isotype (dotted line) was used as control of non-specific fluorescence signals. The data shown are representative of three independent experiments with similar results. PBMCs were cultivated in HEMA condition without EPO for three days and for additional three days in presence of both polyclonal rabbit anti-human TNF-α (1 μg/mL) and rNef/myr or in presence of rNef/myr alone as control of CD36 downregulation. MDMs were then analyzed for CD36 expression by using FICT-conjugated CD36 antibody. (F) The histogram reports the relative fluorescence intensities of CD36 in Nef-treated (solid line), Nef- and anti TNF-α-treated (solid grey histogram) or untreated (dotted line) cells. SYTOX Blue was used to exclude dead cells. (G) The column bar graph represents the MFI of untreated cells (Ctr CD36), Nef-treated cells (Nef CD36), Nef- and anti-humanTNF-α-treated cells (Nef+anti TNF-α CD36) stained with FITC-conjugated CD36 antibody. The results (mean ± standard deviation) are representative of three independent experiments (*p<0.05).