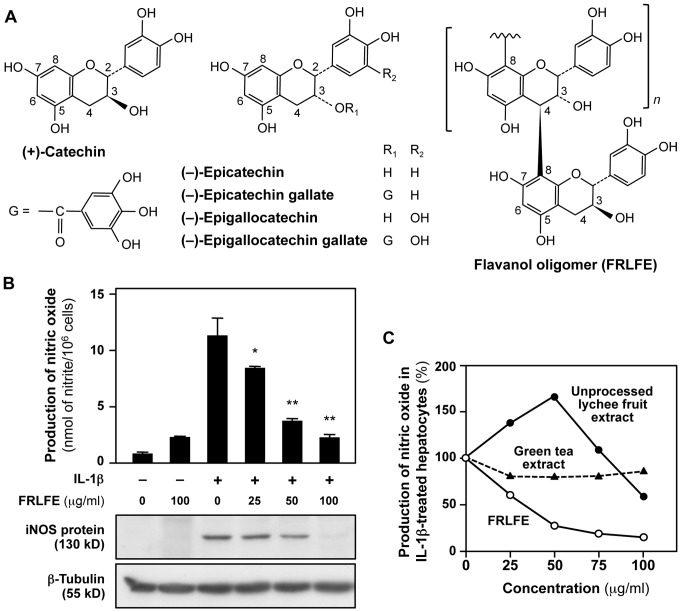
Figure 1. FRLFE suppresses NO production in IL-1β-treated hepatocytes.
(A) Structures of the flavanol monomers in green tea catechins (left and center) and a flavanol polymer in FRLFE (right). Due to the two asymmetric carbons (C-2 and C-3), a flavanol monomer has four diastereoisomers, such as (+)-catechin [2R,3S] (left) and (−)-epicatechin [2R,3R] (center). (−)-Epicatechin gallate (ECG) and (−)-epigallocatechin gallate (EGCG) are galloyl esters of (−)-epicatechin and (−)-epigallocatechin (EGC), respectively. G = galloyl group. A flavanol oligomer from FRLFE (right) was synthesized by creating a covalent bond between (+)-catechin and the lychee fruit procyanidin. (B) FRLFE suppresses the induction of NO production and iNOS protein expression. Rat hepatocytes were treated with or without FRLFE for 8 h. Simultaneously, 0.1 nM IL-1β was added to the cells. The NO levels in the medium were measured in triplicate (mean ± SD), and the cell extracts were immunoblotted with an anti-iNOS or anti-β-tubulin antibody (internal control). *P<0.05, **P<0.01 versus IL-1β alone. (C) FRLFE suppresses the induction of NO production. FRLFE, unprocessed lychee fruit extract, or green tea extract were added to the medium in the presence of 0.1 nM IL-1β. The NO levels in the medium were measured in duplicate (mean). Cytotoxicity was not observed at these concentrations (data not shown).