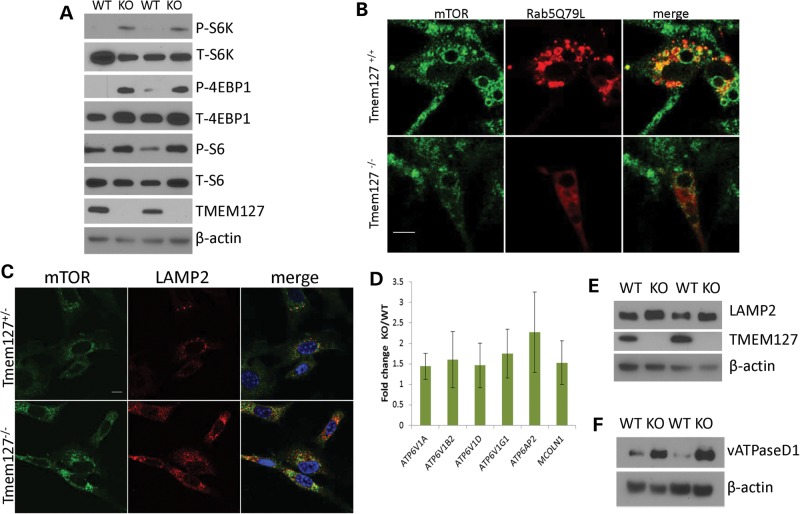
Figure 6.
mTOR association with the lysosomal domain is increased in Tmem127 KO cells. (A) Western blot lysates from two independent Tmem127 null (KO) or WT MEFs probed for phosphorylated (P) or total (T) S6K, S6 and 4EBP1, and also TMEM127 and β-actin. (B) Confocal microscopy images of endogenous mTOR staining (green) of WT (Tmem127+/+) or KO (Tmem127−/−) MEFs after transfection with Flag-Rab5Q79L (red) and amino acid exposure for 15 min following a 50 min amino acid deprivation. Merged images are shown in yellow. Scale bar is 10 µm. Quantification of colocalization of mTOR/Rab5Q79L is shown in Supplementary Material, Figure S6. (C) Confocal microscopy of endogenous mTOR (green), LAMP2 (red) and merged images in KO or WT MEFs maintained under steady-state culture conditions (Supplementary Material, Fig. S7a shows quantification of the degree of colocalization). Scale bar is 10 µm; (D) Real-time PCR quantification of lysosomal genes vacuolar ATPase (vATPase) subunits V1a, V1b2, V1d, V1g1, AP2 and the gene encoding for Mucolipin 1 (Mcoln1) in MEFs. Results represent the fold change of KO in relation to WT MEFs, generated from triplicate data (±SEM) from experiments performed in independent pairs of MEFs. (E) Western blot of lysates from two independent Tmem127 KO or WT MEF pairs, probed with LAMP2, TMEM127 and β-actin. (F) Western blot of MEF lysates from two independent Tmem127 KO or WT MEF pairs, probed with vATPase D1, TMEM127 and β-actin.