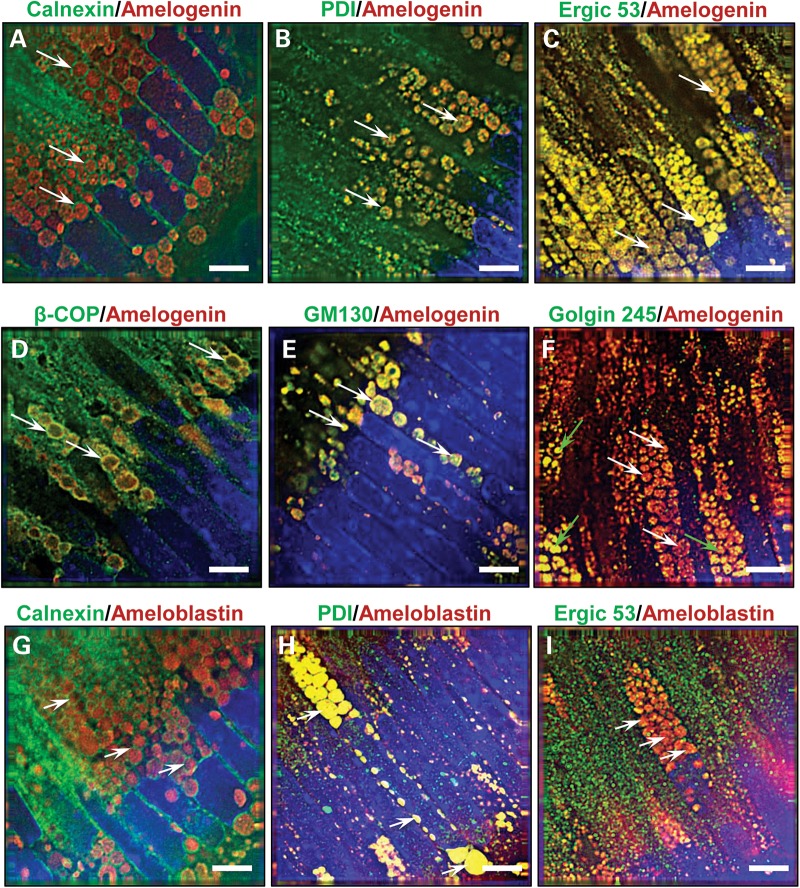
Figure 2.
Characterization of the cytoplasmic vesicles observed in affected male mice. (A–F) Dual immuno-staining for amelogenin (red fluorescence) or (G–I) ameloblastin (red fluorescence) and components of the secretory pathway (green fluorescence) in affected male mice (n = 4) secretory ameloblasts indicate arrest of amelogenin trafficking at a stage prior to the trans-Golgi. (A) The ER membrane protein calnexin circumscribes the amelogenin-containing vesicles (arrows), while the ER luminal molecule protein disulphide isomerase (B) co-localizes with amelogenin within the vesicles (arrows). (C) Immuno-reactivity for the ER/Golgi intermediate compartment molecule Ergic 53 also co-localizes with amelogenin in the vesicles (arrows), whereas (D) β-COP, a component of COP1 vesicles found at the cis-Golgi and ER/Golgi intermediate compartment, co-localizes with amelogenin at the periphery of the vesicles (arrows). (E) The cis-Golgi protein GM130 co-localizes with amelogenin within the vesicles (arrows). (F) Golgin 245, a trans-Golgi protein, showed a variable pattern of immuno-reactivity; in most instances, little amelogenin co-localization was observed in the vesicles (white arrows), although, less typically, partial or complete co-localization was seen (green arrows). (G) Dual immunofluorescence for ameloblastin and the ER membrane protein calnexin indicates that calnexin circumscribes the inclusions (arrows). (H) Similar analyses using antibodies directed against ameloblastin and the ER luminal molecule protein disulphide isomerase (PDI) indicate co-localization within the vesicles (arrows). (I) In contrast, dual immunofluorescence with ameloblastin and Ergic 53, a protein found in the ER/Golgi intermediate compartment, demonstrates distinct localization within the ameloblasts (arrows). Nuclei are stained with DAPI (blue). Scale bars: 5 μm.