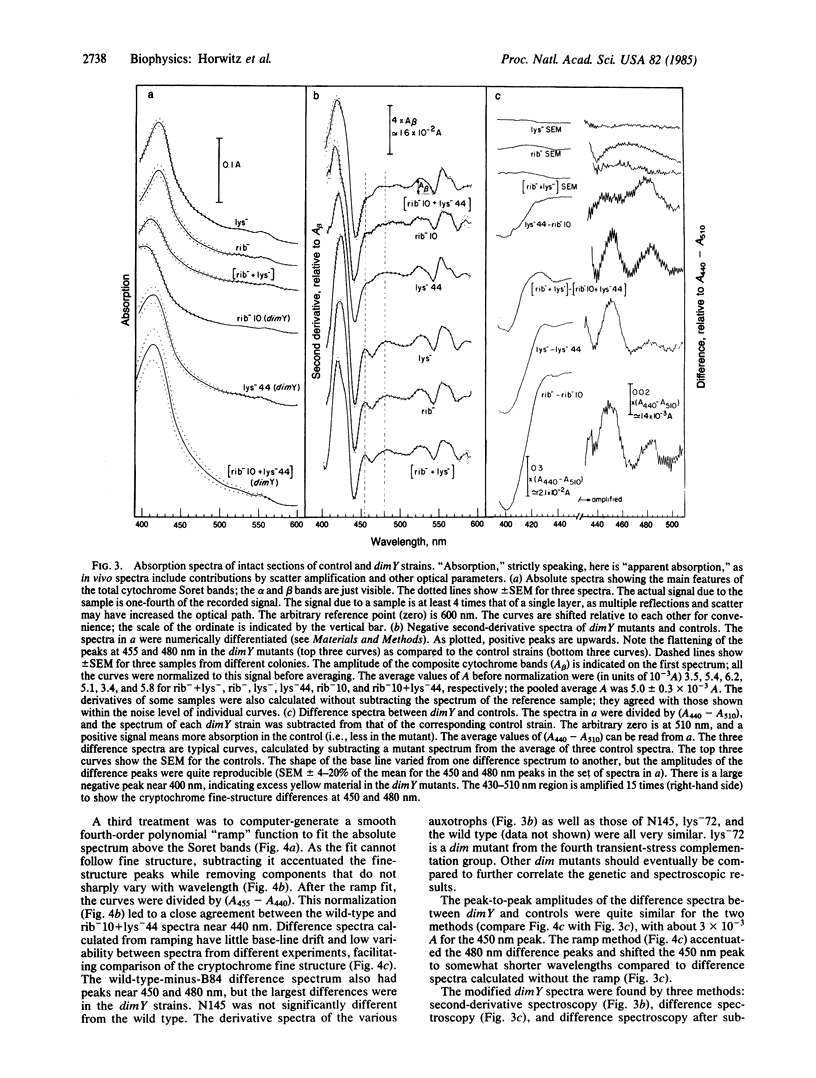
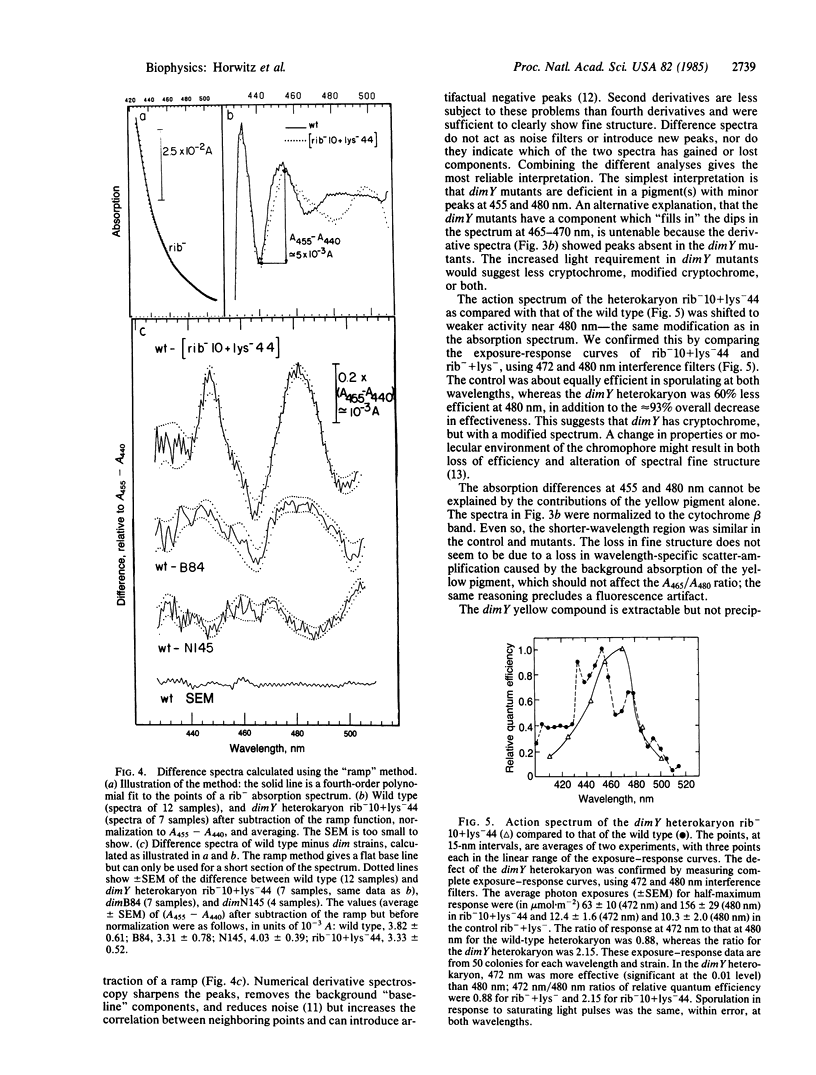
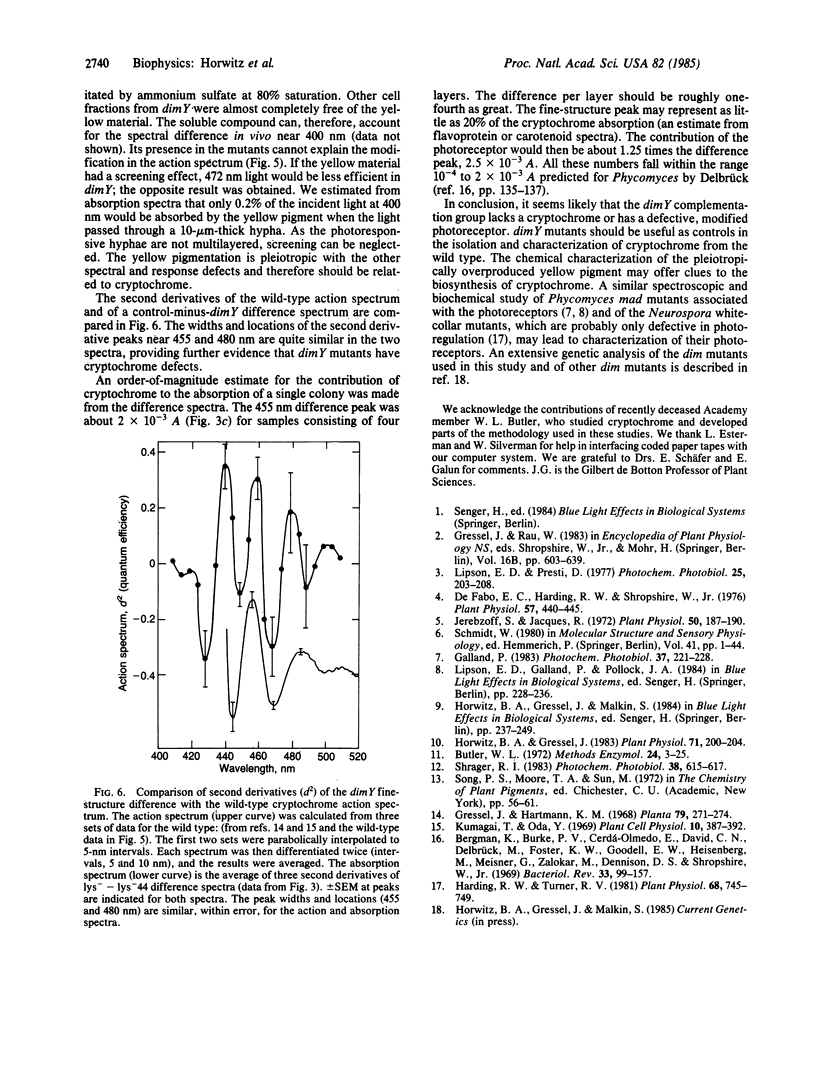
Abstract
Mutants of Trichoderma harzianum that are defective in blue-light-induced sporulation were induced by nutritional stresses as an alternative to light. These mutants may be modified in the putative photoreceptor pigment “cryptochrome” or in an early transduction component. dim (dimsighted) mutants induced by a short transient stress were mapped into four complementation groups and were chosen for study of pigment deficiencies by in vivo absorption spectroscopy. Mutants rib-10 and lys-44 in the dimY complementation group had altered in vivo absorption spectra in the blue region. Difference spectra obtained by subtracting dimY spectra from that of the wild type had difference bands with peaks at 455 and 480 nm. The similarity between the in vivo difference spectra and the action spectrum for sporulation in wild-type Trichoderma suggests that the mutants lack cryptochrome or have a defective cryptochrome. The decrease in photoresponse as well as the modification of the action spectrum near 480 nm in a dimY mutant support these suggestions. Both dimY mutants pleiotropically accumulate a yellow water-soluble pigment absorbing at wavelengths lower than the blue maxima of cryptochrome; this yellow pigment may be related to cryptochrome.
Keywords: photomorphogenesis, blue light, in vivo spectroscopy, developmental genetics
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergman K., Burke P. V., Cerdá-Olmedo E., David C. N., Delbrück M., Foster K. W., Goodell E. W., Heisenberg M., Meissner G., Zalokar M. Phycomyces. Bacteriol Rev. 1969 Mar;33(1):99–157. doi: 10.1128/br.33.1.99-157.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler W. L. Absorption spectroscopy of biological materials. Methods Enzymol. 1972;24:3–25. doi: 10.1016/0076-6879(72)24052-6. [DOI] [PubMed] [Google Scholar]
- De Fabo E. C., Harding R. W., Shropshire W. Action Spectrum between 260 and 800 Nanometers for the Photoinduction of Carotenoid Biosynthesis in Neurospora crassa. Plant Physiol. 1976 Mar;57(3):440–445. doi: 10.1104/pp.57.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding R. W., Turner R. V. Photoregulation of the Carotenoid Biosynthetic Pathway in Albino and White Collar Mutants of Neurospora crassa. Plant Physiol. 1981 Sep;68(3):745–749. doi: 10.1104/pp.68.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. A., Gressel J. Elevated riboflavin requirement for postphotoinductive events in sporulation of a trichoderma auxotroph. Plant Physiol. 1983 Jan;71(1):200–204. doi: 10.1104/pp.71.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerebzoff S., Jacques R. Equal Quantal Spectra for the Effect of Light on the Growth of Conidiophores and for the Induction of a Circadian Rhythm of Zonation in Sclerotinia fructicola (Wint.) Rehm. Plant Physiol. 1972 Jul;50(1):187–190. doi: 10.1104/pp.50.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]



