Figure 2.
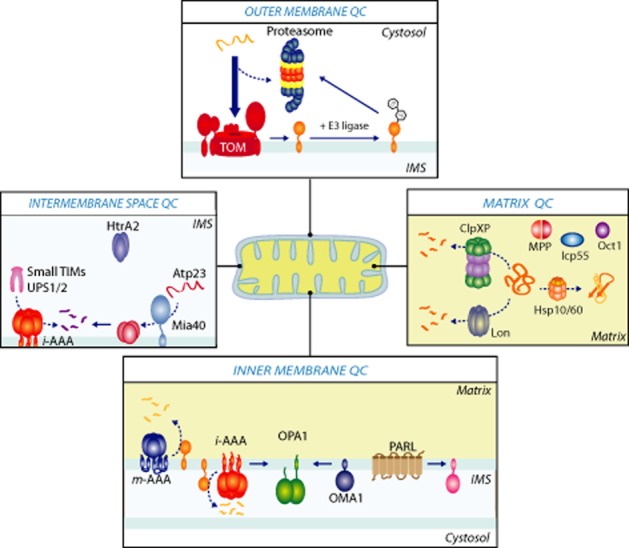
Mitochondrial protein quality control. Each mitochondrial compartment has its own quality control machinery. Outer mitochondrial membrane proteins can be ubiquitinated by E3 ligases at the mitochondria surface and are degraded through the ubiquitin/26S proteasome system. Premature precursors in the cytosol can also be targets for the proteasome, prior to being imported into mitochondria. In the mitochondrial matrix, chaperones belonging to Hsp60 and Hsp70 families facilitate protein translocation and folding reactions. Precursor processing such as removal of the N-terminal presequence is mediated by the MPP. Two additional proteases, Icp55 and Oct1, play a role in processing steps post MPP cleavage. Damaged proteins within the mitochondrial matrix are preferentially degraded by the Lon protease and the ClpXP complex. Protein quality control in the inner membrane is primarily monitored by two AAA proteolytic complexes, the i-AAA protease and m-AAA protease with their respective active sites facing the intermembrane space (i-AAA) or matrix (m-AAA). The i-AAA protease in conjunction with the inner membrane metallopeptidase OMA1 regulates mitochondrial morphology by proteolytic processing of the dynamin-like GTPase OPA1. The rhomboid serine protease, PARL, mediates cleavage of outer membrane kinase Pink1 in healthy mitochondria. Finally, in the intermembrane space, the oxidoreductase Mia40 can serve as a chaperone for the import of substrates such as the metallopeptidase Atp23. The i-AAA protease and Atp23 can degrade intermembrane space proteins, such as Ups1, whereas the i-AAA alone is responsible for the turnover of Ups2 and the small TIM chaperones, Tim9 and Tim10. Finally, HtrA2 encodes a serine protease and has been implicated in both apoptosis and Parkinson's disease.
