Figure 4.
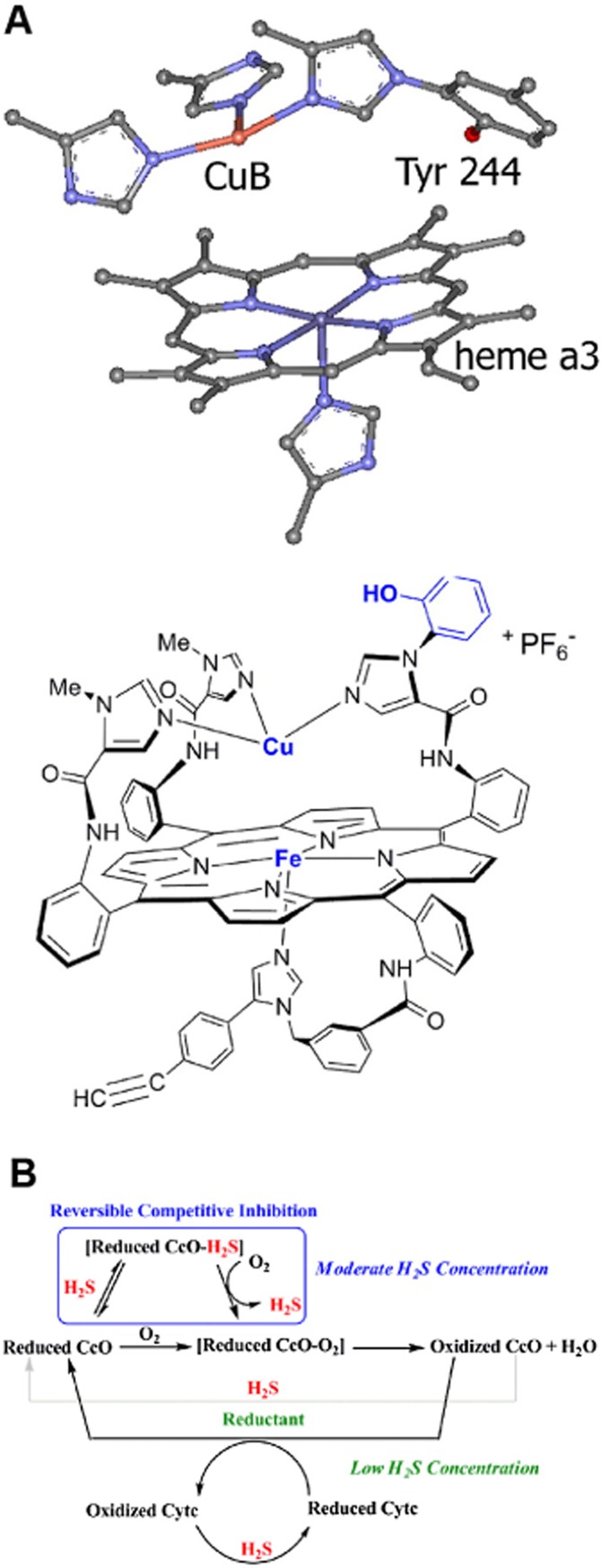
Mechanisms of inhibition of cytochrome c oxidase by H2S. (A) Active site structures of cytochrome C oxidase (CcO, top) and its synthetic model (Fe Cu and phenol analogue) used to study the mechanism of its inhibition by sulfide (Collman et al., 2009). (B) Schematic representation of the possible roles of H2S affecting CcO activity, as derived from the synthetic model shown in part A. The box indicates that H2S binds to the reduced CcO active site, but can be subsequently replaced by O2. This inhibition is hypothesized to occur at moderate H2S concentrations. At lower concentrations, H2S does not compete with the substrate O2 but can still reduce CcO's active site and/or cytochrome c (Cytc) during catalytic O2 reduction. From Collman et al. (2009), reproduced by permission.
