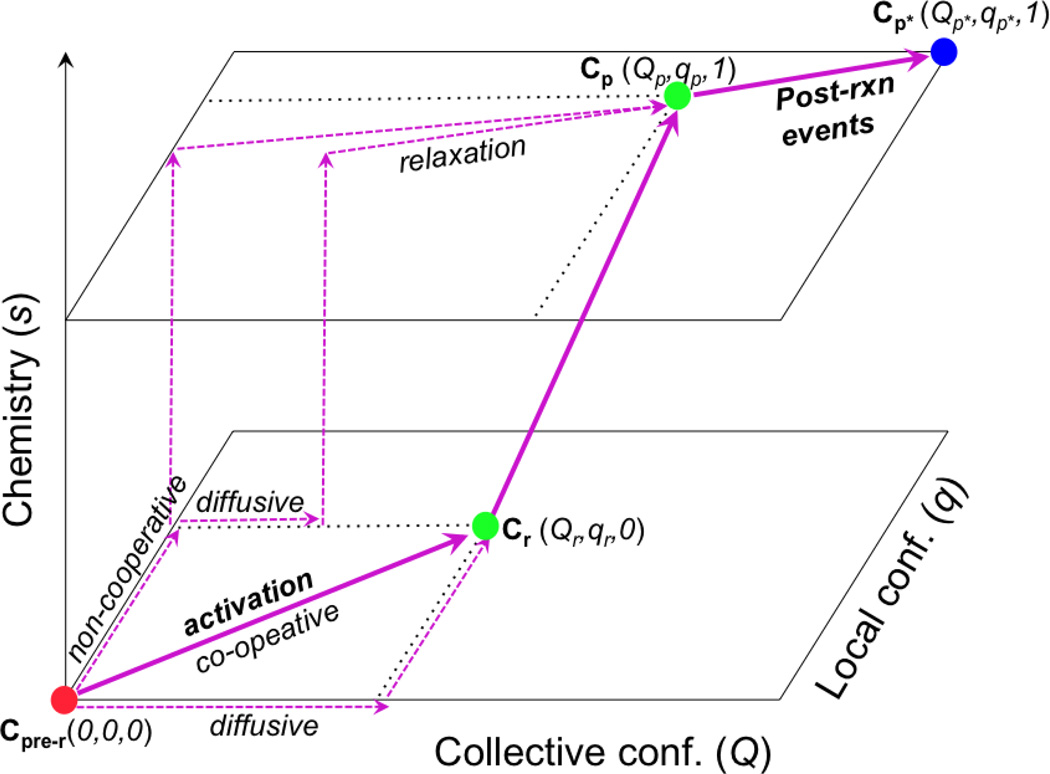
Figure 1.
A schematic sketch that illustrates the coupling between multi-scale structural changes (both collective and local conformational transitions) and chemistry in enzymes. The filled circles indicate kinetic states with different conformational (Q, q) and chemical (s) coordinates; the thick arrows indicate the dominant pathways that feature highly co-operative conformational transitions, while the dashed arrows indicate hypothetic pathways that are less co-operative and presumably have less flux. The “activation” process that goes from a pre-reactive conformation (Cpre−r) to a reactive conformation (Cr) may correspond to an open/close transition of an enzyme active site (e.g., in adenlyate kinase) or the recovery stroke of myosin (see below); this “activation” process is the focus of this article. The chemical step may also involve structural transitions of multiple scales, as discussed in other studies. Finally, the Cp → Cp* transition corresponds to structural changes following the chemical step in the catalytic cycle, such as opening of the active site for product release or the power stroke of myosin due to actin binding and release of inorganic phosphate.