Figure 3).
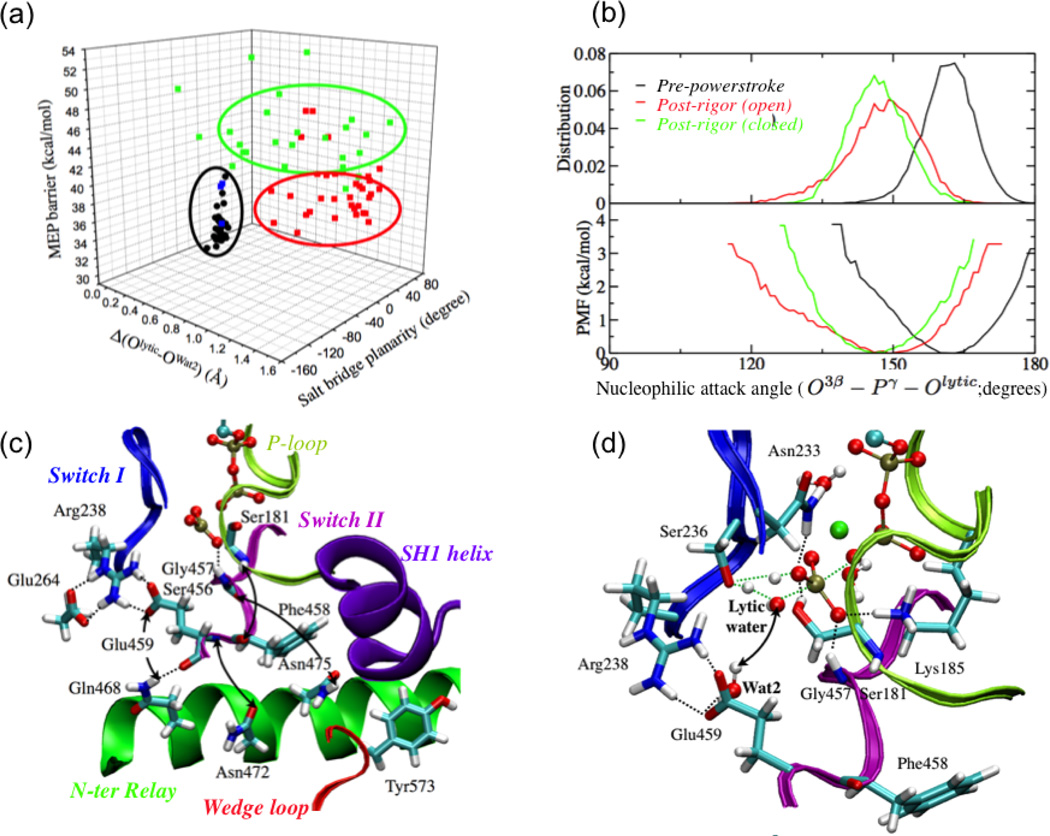
The dependence of ATPase activity of the myosin on the structural state of the motor domain, i.e., the mechanochemical coupling in myosin. (a) Minimum energy path (MEP) barriers for the first step of ATP hydrolysis calculated starting from snapshots collected from equilibrium simulations of the pre-powerstroke state and a closed post-rigor structure (see text). The barriers are plotted against the Arg238-Glu459 salt bridge planarity and the differential distance between the lytic water and Wat2 (see panel d) in the reactant and transition state. The black dots indicate data for the pre-powerstroke state; the blue, green and red set indicate data from the closed post-rigor simulations with different behaviors of Wat241. Note that the MEP barriers are systematically higher than the free energy barrier due to the lack of sampling specific local rearrangements; see Ref.41 for discussions. (b) Comparison of the distribution and corresponding PMF for the nucleophilic attack angle based on equilibrium simulation for the pre-powerstroke state and two post-rigor structures. (c) Key hydrogen-bonding interactions in the active site region of the closed post-rigor structure. The arrows indicate interactions that are broken when SwII is displaced to close the active site in the post-rigor state; i.e., rearrangements in the N-terminus of the relay helix and wedge loop are required to form the stable active site as in the pre-powerstroke state. (d) A representative active site structure for the ATP hydrolysis transition state with a twisted Arg238-Glu459 salt-bridge configuration. The notable feature is that Wat2 in the active site remains hydrogen bonded to Glu459 and therefore does not provide the critical stabilization for the transition state.