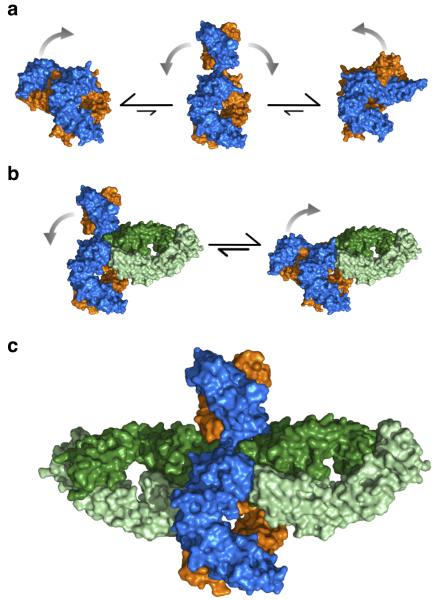
Figure 4. Proposed mechanism of IgE-Fc flexibility and aεFab binding in solution.
(a) IgE-Fc is predominantly bent in solution, but is capable of adopting transiently extended conformations through which the (Cε2)2 domains can flip from one side of the molecule to the other. (b) aεFab1 engages one of the binding sites of IgE-Fc restricting its range of flexibility, but IgE-Fc is neither exclusively bent nor exclusively extended. (c) aεFab2 engages the extended form of IgE-Fc, capturing the molecule in this otherwise transiently occupied conformation. Colored as in Fig. 1.