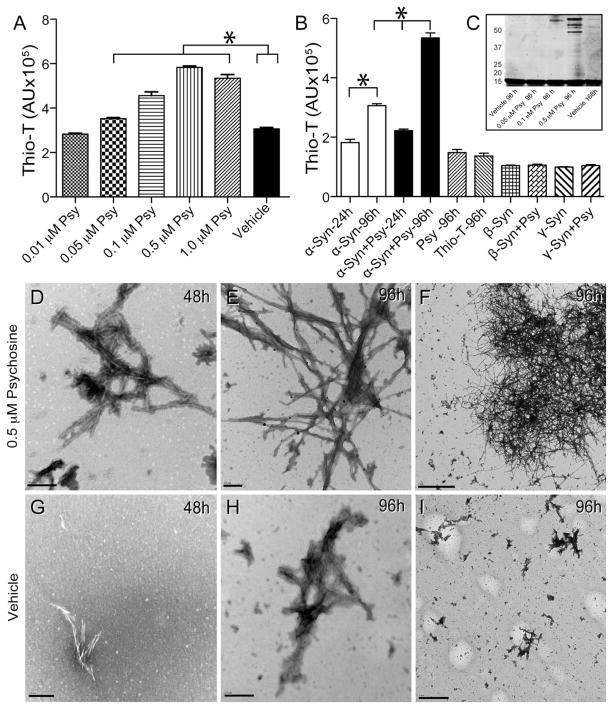
Figure 6. Psychosine increases the rate of α-synuclein fibrillation.
A, B) An in vitro fibrillization assay was performed by incubating recombinant human α-synuclein (α-syn) in the presence of increasing concentrations of psychosine (Psy) or vehicle (0.005% ethanol/PBS) and measuring emitted fluorescence from thioflavin-T (thio-T) for up to 96h. Significant (ANOVA, p<0.05) increases of thioflavin-T fluorescence were measured in the presence of psychosine in a dose dependent manner. Psychosine alone did not show fluorescence above background levels from thioflavin-T. Psychosine did not cause fibrillization of either β- or γ-synucleins. C) Aliquots of fibrillizated α-synuclein in the presence or absence of different concentrations of psychosine were analyzed by gel separation. Protein bands were developed using Sypro fluorescent stain and scanned using a Typhoon fluorometer. D–I) Transmission electron microscopy detected the presence of abundant fibrillizated α-synuclein in the presence of 0.5 μM psychosine 48 hours after treatment (D). Fibrillization of α-synuclein was more abundant at 96 h of incubation in the presence of psychosine (E, F). α-Synuclein formed straight groups of filaments approximately 50–75nm in width and also formed larger, branched aggregates approximately 300–700 nm in length (E, F). Smaller and less abundant α-synuclein fibrils were detected in vehicle-treated conditions (G–I). Scale bars: D, E, G, H, 200 nm; F, 2μm; I, 1μm.