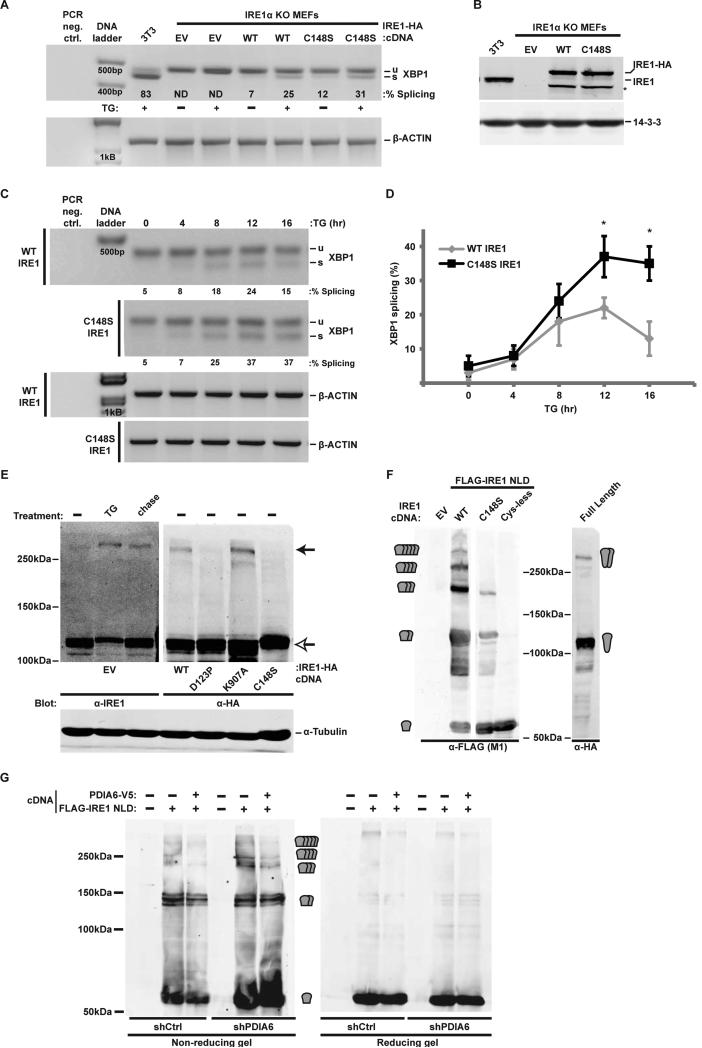
Figure 5. The reactivity of Cys148 is important for the activation kinetics of IRE1.
(A) IRE1 knockout (KO) MEFs stably complemented with WT or C148S IRE1-HA were treated with 300nM TG for 2hrs. RNA samples were then assayed for XBP1 splicing. Percentage of splicing is indicated in each lane. TG-treated 3T3 served as positive controls. EV, empty cDNA vector. ND, not detectable.
(B) Cell lysates from the same samples used in (A) were immunoblotted to determine the level of expression of the rescuing IRE1-HA. 3T3 lysates served as reference for endogenous IRE1 expression. Note that the expression of each rescuing protein as well as the endogenous IRE1 are similar. *, unknown product of the expression plasmid, presumably a cleaved IRE protein.
(C) Extent of XBP1 splicing in IRE1-HA-rescued IRE1 KO MEFs was determined upon persistent exposure to 300nM TG for the indicated times. β-actin served as loading control.
(D) Means±SD of experiments as in (C) are plotted (*, p ≤ 0.05; n=3).
(E) A C148-dependent disulfide forms during activation of IRE1. 293T cells were transfected with either empty vector (EV) or with the indicated IRE1-HA cDNAs (under control of the pCAX promoter). Cells were treated with 100nM TG for 2hrs, followed by 16hr chase in fresh medium where indicated. Reactive cysteines were alkylated with NEM prior to cell lysis and endogenous or HA-tagged exogenous IRE1 were detected by non-reducing gel. α-tubulin (which does not contain reactive cysteines) was used as control for the electrophoretic shift.
(F) 293T cells were transiently transfected with full length IRE1 or with FLAG-tagged IRE1 luminal domain (NLD) that was either WT, C148S or lacked all three cysteines (C109/148/332S). Expression of all NLDs was limited to the ER with a C-terminal KDEL peptide. Protein extracts were resolved by non-reducing PAGE and analysed by immunoblot. EV, empty vector transfection. The predicted sizes of monomeric NLD or multimeric complexes are indicated at the sides of the blots.
(G) shCtrl and shPDIA6 293T cells were transfected with WT IRE1 NLD or mock transfected. Protein extracts were analysed as in F. Co-expressed PDIA6-V5 complemented the silenced enzyme in shPDIA6 cells in reducing multimer formation.
See also Fig. S5.