Significance
The value and salience of predictive cues are important signals for regulating approach–avoidance behavior and attentional processing, respectively. However, the two signals often are confounded in studies of decision-making. Indeed, recent results suggest that neural signals in the primate posterior parietal cortex (PPC) which previously were thought to encode value actually reflect salience. This finding has created considerable uncertainty about previously identified value signals. Here we experimentally dissociate value and salience and use pattern-based functional MRI to demonstrate distinct encoding of both signals in the PPC, thereby reinforcing the earlier reports of value in the PPC. Moreover, we show that the orbitofrontal cortex encodes the predicted value of appetitive and aversive outcomes on a common neural scale.
Keywords: reward, punishment, decision-making, attention, MVPA
Abstract
A large body of evidence has implicated the posterior parietal and orbitofrontal cortex in the processing of value. However, value correlates perfectly with salience when appetitive stimuli are investigated in isolation. Accordingly, considerable uncertainty has remained about the precise nature of the previously identified signals. In particular, recent evidence suggests that neurons in the primate parietal cortex signal salience instead of value. To investigate neural signatures of value and salience, here we apply multivariate (pattern-based) analyses to human functional MRI data acquired during a noninstrumental outcome-prediction task involving appetitive and aversive outcomes. Reaction time data indicated additive and independent effects of value and salience. Critically, we show that multivoxel ensemble activity in the posterior parietal cortex encodes predicted value and salience in superior and inferior compartments, respectively. These findings reinforce the earlier reports of parietal value signals and reconcile them with the recent salience report. Moreover, we find that multivoxel patterns in the orbitofrontal cortex correlate with value. Importantly, the patterns coding for the predicted value of appetitive and aversive outcomes are similar, indicating a common neural scale for appetite and aversive values in the orbitofrontal cortex. Thus orbitofrontal activity patterns satisfy a basic requirement for a neural value signal.
The value of predictive cues can be used to guide approach–avoidance behavior. Approach and avoidance responses are proportional to the appetitive (positive) and aversive (negative) value of the cues, respectively. On the other hand, based on empirical and theoretical considerations (1–3) the absolute value (i.e., the salience) of a cue determines the amount of attention that a stimulus captures to facilitate further processing. Hence, in contrast to value, salience increases not only with the magnitude of reward but also with the magnitude of punishment (4, 5).
Electrophysiological recordings in animals suggest that value is encoded in the firing rates of posterior parietal and orbitofrontal neurons (6–18). A large body of evidence from human imaging studies also suggests signatures of appetitive value in these regions (19–36). However, value and salience are perfectly correlated when appetitive stimuli are investigated in isolation (37). That is, if a signal increases with increasing reward, we need to know how it behaves with increasing punishments to decide whether it is coding for value or salience. Specifically, if the signal decreases with increasing punishment, it truly reflects value. However, if the signal also increases with increasing punishment, it reflects salience (Fig. 1C). Thus, value signals identified using only appetitive (or only aversive) stimuli could be explained equally well in terms of value or salience.
Fig. 1.
Task structure, stimuli, and behavioral results. (A) Structure and timing of the task. Associations between buttons and ratings were randomized in each trial. (B) Example of cue–outcome associations (actual associations were randomized across subjects). (C) Dissociation of value and salience using appetitive and aversive outcomes. Salience corresponds to the absolute value of predicted outcomes. Note that the use of only appetitive (or aversive) outcomes alone would not allow the dissociation of value and salience. (D) Effects of value and salience on RTs for the ratings. Bars represent standardized regression coefficients from individual multiple regressions, averaged across subjects. Error bars depict SEM for n = 30. Asterisks indicate significant effects at P < 0.05 (one sample t test).
Indeed, neurons in the lateral intraparietal area (LIP), which have long been thought to signal decision values (6–9), have been shown recently to signal salience (37). This result has created considerable uncertainty regarding previous findings on the neural coding of value, not only in the posterior parietal cortex (PPC) but also in the orbitofrontal cortex (OFC). Here, to assess the nature of anticipatory value and salience signals in the human PPC and OFC, we use a noninstrumental outcome-prediction task and multivoxel pattern-based functional MRI (fMRI). This analysis technique combines the activity of multiple voxels and can be used to reveal signals encoded in intercalated neuronal populations (SI Discussion). To dissociate value and salience signals, the current task involves distinct stimuli predicting small or large appetitive or aversive outcomes.
We carry out three complementary analyses on the fMRI data aiming to determine where and how value and salience signals are represented. First, by searching for multivoxel response patterns that code for one or the other variable, we identify brain regions carrying information about the two signals. Second, in regions encoding value or salience, we test whether these multivoxel patterns code for value differences within the appetitive and the aversive domain (thus reflecting graded value and not only categorical valence) and whether appetitive and aversive cues contribute similarly to the observed neural encoding. Third, we ask whether the multivoxel ensemble codes of value or salience are similar for appetitive and aversive values. In other words, we test whether we can predict the value of an aversive cue from multivoxel response patterns coding for the value of appetitive cues.
Results
Behavioral Results.
We used a simple noninstrumental outcome-prediction task (Fig. 1A) in which visual cues deterministically (100% cue–outcome contingency) predict the gain or loss of small or large amounts of money (i.e., 0.50 € or 5.00 €). Here we assume value to increase linearly with nominal monetary magnitude, as usually holds true for small amounts or intervals (SI Discussion) (38). Two sets of cues were associated with the four possible outcomes (−5.00 €, −0.50 €, 0.50 €, and 5.00 €) (Fig. 1B), such that each outcome was predicted by two different visual cues. We used two sets to control for the sensory properties of the cues (29). Subjects (n = 30) had to indicate the predicted outcome after a variable delay and before the actual outcome was shown. On average, subjects were correct in predicting the outcome on almost every trial [average % correct = 95.54; one sample t test against chance (four options, chance = 25%), t29 = 132.60, P < 0.001]. However, note that the cue–outcome pairing was purely noninstrumental, and thus outcomes were independent of the correctness of the response.
By using both appetitive and aversive cues, this task allows for linearly independent (i.e., uncorrelated) levels of value and salience (Fig. 1C). We used response time (RT) to search for behavioral effects of value and salience. Specifically, to estimate and compare the behavioral effects of value and salience, we applied subjectwise multiple regression models (Materials and Methods). On average, we observed significant negative regression coefficients for both value (one-sample t test, t29 = −2.82, P = 0.009) and salience (t29 = −2.72, P = 0.011), indicating that high levels of value and salience led subjects to respond faster. Moreover, the effects of value and salience on RT did not differ (paired t test on standardized regression coefficients, t29 = 0.29, P = 0.78), suggesting that both variables affected behavior to a comparable degree. The independent influence of value and salience on RT should result in a magnitude by valence interaction and a main effect of magnitude, and our data confirmed these predictions (Fig. S1 and SI Results).
Identifying Brain Regions Coding for Value and Salience.
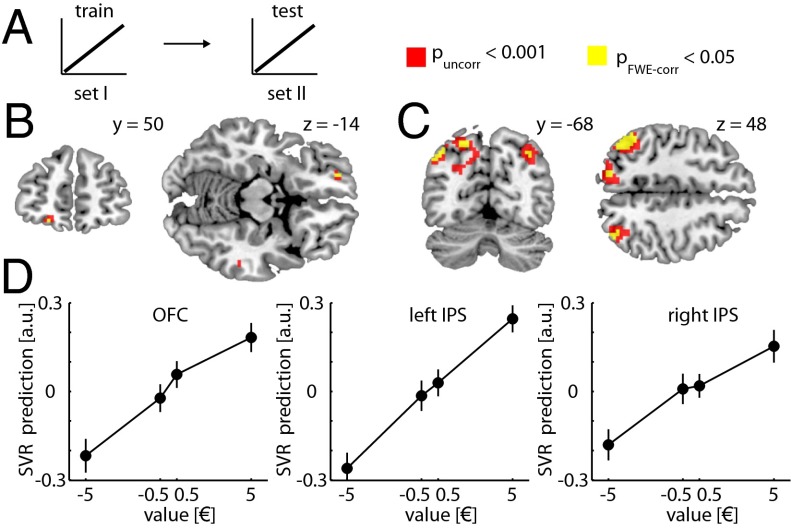
First, we searched for brain regions coding for the predicted value of the cues independent of their sensory properties. To do so, we used a searchlight cross-decoding approach using linear support vector regression (SVR) and leave-one-out cross-validation (Materials and Methods). In brief, for each searchlight (radius: three voxels), we trained an SVR based on the multivoxel response patterns evoked by the cues in set I (using the value of appetitive and aversive cues as labels, i.e., −5.00 €, −0.50 €, 0.50 €, and 5.00 €) and predicted the value of the cues in set II based on their corresponding response patterns (Fig. 2A). We also performed the same analysis in the opposite direction by training on cues from set II and testing on cues from set I (results represent the average). Across subjects, we find significant information about the predicted value of the cues in the central OFC [Montreal Neurological Institute (MNI) coordinates: x, −21; y, 53; z, −14; t29 = 3.83, familywise error-corrected P value (PFWE-corr) = 0.048] (Fig. 2B). Moreover, we also find significant information about value in superior regions of the PPC along the intraparietal sulcus (IPS) (left IPS: −48, −61, 46, t29 = 6.70, PFWE-corr < 0.001; right IPS: 39, −73, 43, t29 = 4.65, PFWE-corr = 0.015) (Fig. 2C). Thus, multivoxel patterns in these regions can be used to make predictions about the value of the predictive cues (Fig. 2D).
Fig. 2.
Decoding value information. (A) Schematic of the decoding analysis. SVR models were trained on data from set I and tested on data from set II (and vice versa) across appetitive and aversive cues. (B) Coronal (Left) and transversal (Right) sections depicting regions in the OFC with significant information about the value of the cues. (C) Coronal (Left) and transversal (Right) sections depicting regions in the superior PPC with significant information about the value of the cues. For display purposes, the t-map (one-sample t test) is thresholded at P < 0.05FWE-corr (yellow) and Puncorr < 0.001 (red). (D) For illustration proposes, labels predicted by the SVR model are plotted as a function of the actual values in the test dataset. SVR outputs from peak searchlights are normalized and averaged across cross-validation steps and subjects. Error bars depict SEM for n = 30.
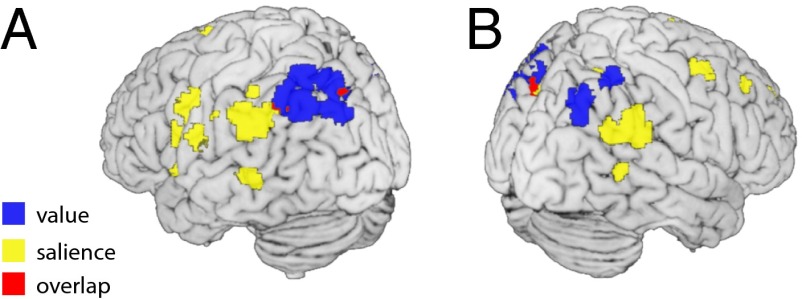
Second, we searched for brain regions that encode the salience of the cues independent of their sensory properties. To do so, we applied the pattern-based analysis as described above, but this time we used salience (absolute value) as the label for training and testing the SVR (i.e., 5.00 €, 0.50 €, 0.50 €, and 5.00 €) (Fig. 3A). We found significant information about the salience of the cues in the PPC, specifically in inferior regions such as the temporoparietal junction (TPJ) (left TPJ: −60, −43, 31, t29 = 4.65, PFWE-corr = 0.019; right TPJ: 60, −49, 34, t29 = 5.58, PFWE-corr = 0.002) (Fig. 3B) but also a trend in the right IPS (24, −52, 58, t29 = 4.19, PFWE-corr = 0.056) and more medial areas extending into the precuneus (−12, −73, 43, t29 = 4.36, PFWE-corr = 0.037). Finally, we also find significant salience information in the anterior cingulate cortex (ACC) (6, 41, 25, t29 = 4.10, PFWE-corr = 0.029) (Fig. 3C). Taken together, the results of these analyses show that value and salience signals are both encoded in the PPC, albeit in different subregions spanning a superior–inferior gradient (Fig. 4 and Fig. S2). The superior PPC primarily encodes value but also shows a trend for salience, whereas the inferior PPC encodes salience only.
Fig. 3.
Decoding salience information. (A) Schematic of the decoding analysis. SVR models were trained on data from set I and tested on data from set II (and vice versa) across appetitive and aversive cues. (B) Coronal (Left) and sagittal (Right) sections depicting regions in the inferior PPC with significant information about the salience of the cues. (C) Coronal (Left) and sagittal (Right) sections depicting regions in the ACC with significant information about the salience of the cues. For display purposes, the t-map (one-sample t test) is thresholded at P < 0.05FWE-corr (yellow) and Puncorr < 0.001 (red). (D) For illustration proposes, labels predicted by the SVR model are plotted as a function of the actual values in the test dataset. SVR outputs from peak searchlights are normalized and averaged across cross-validation steps and subjects. Error bars depict SEM for n = 30.
Fig. 4.
Value and salience signals in the posterior parietal cortex. Surface plots of the left (A) and right (B) hemisphere depict regions with significant information about value (blue), salience (yellow), and their overlap (red). For illustrative purposes, t-maps (one-sample t tests) are thresholded at Puncorr < 0.001.
Decoding the Value and Salience of Appetitive and Aversive Cues.
In a post hoc analysis, we addressed three further issues. First, we asked whether the neural patterns identified above indeed code for the graded value of both appetitive and aversive predicted outcomes and not just the general difference between aversive and appetitive domains (categorical valence). That is, we tested whether these patterns encode information not only about the sign of the predicted outcome (i.e., appetitive vs. aversive) but also about the degree to which predicted outcomes are appetitive or aversive (i.e., low vs. high), as would be expected from a value-coding region. Second, we wanted to rule out the possibility that only appetitive or only aversive predicted outcomes contributed to the encoding of either value or salience. Third, we asked whether appetitive and aversive values contributed differentially to the neural encoding of value.
To address these issues, we used the SVR model that was trained on the value of all cues from set I and tested it separately on the value of appetitive and aversive cues from set II (Fig. 5A), and vice versa by training on set II and separately testing on appetitive and aversive cues from set I (results are averaged across both directions). This procedure results in two accuracy measures, one reflecting the encoding of appetitive values and the other the encoding of aversive values (Fig. 5A). We performed this analysis on the individual value-coding patterns identified above using the individual peak-accuracy searchlights (radius: three voxels) in value-coding regions. In particular, we used the individual peak-accuracy searchlights in a 12-mm sphere surrounding the group peak voxel in the OFC and left and right IPS. (Changing the size of this search sphere to 8–14 mm led to qualitatively similar results.) All accuracies for testing the SVR model individually on appetitive and aversive cues were significant in all regions (one-sample t tests, all Ps < 0.001). This result indicates that the multivoxel response patterns identified above encode not only the sign of the predicted outcome but also the degree to which predicted outcomes are appetitive or aversive. Also, these results demonstrate that the value codes indeed are not driven only by aversive cues or only by appetitive cues.
Fig. 5.
Contribution of appetitive and aversive cues to neural encoding of value and salience. (A) (Upper) The decoding analysis. SVR models were trained on the value of all cues from set I and tested on the value of either only appetitive (green) or only aversive cues (red) from set II (and vice versa). (Lower) Bars reflect average accuracy (Fisher’s z-transformed correlation) for appetitive (green bars) and aversive cues (red bars) in the OFC and left and right IPS (superior PPC). All accuracies are significant at P < 0.001 (one-sample t test). (B) (Upper) The decoding analysis. SVR models were trained on the salience of all cues from set I and tested on the salience of only appetitive (green) or only aversive cues (red) from set II (and vice versa). (Lower) Bars reflect average accuracy for appetitive (green bars) and aversive cues (red bars) in the ACC and left and right TPJ (inferior PPC). All accuracies are significant at P < 0.001 (one-sample t test). Error bars depict SEM for n = 30, n.s. indicates nonsignificant differences (paired t tests, all Ps > 0.19).
Next, we tested whether appetitive and aversive values contributed differentially to the neural encoding of value. A two-way (region-by-valence) ANOVA with repeated measures on accuracy revealed no significant main effect of region (F2,58 = 1.79, P = 0.18) or valence (F1,29 = 2.14, P = 0.15) and no significant region-by-valence interaction (F2,58 = 0.54, P = 0.59). This finding suggests that appetitive and aversive values are not differentially encoded. However, it is based on a null result and thus should not form the basis of strong conclusions.
We performed a parallel analysis in the salience-coding regions defined above (ACC and left and right TPJ) to estimate the encoding of appetitive and aversive salience information (Fig. 5B). In all regions, accuracies for appetitive and aversive predicted outcomes were significant (all Ps < 0.001), demonstrating that salience encoding was not driven only by appetitive or aversive cues. Moreover, a two-way (region-by-valence) ANOVA with repeated measures on accuracy revealed no significant effect of region (F2,58 = 0.84, P = 0.45), valence (F1,29 = 1.84, P = 0.19), or their interaction (F2,58 = 0.97, P = 0.39), suggesting that salience is not differentially encoded in appetitive and aversive domains. Note, however, that this finding also is based on a null result and should be treated with caution.
Common Neural Scale for Appetitive and Aversive Value.
In a further post hoc analysis we tested whether the value of appetitive and aversive cues is represented by a similar neural code. If the brain represents values spanning the full range from negative (aversive) to positive (appetitive) levels on one common neural scale, it should be possible to decode the value of aversive cues based on the knowledge we have about the neural encoding of appetitive cues. In other words, the difference between something slightly good (0.50 €) and something very good (5.00 €) should result in a multivoxel response pattern similar to that evoked by the difference between something very bad (−5.00 €) and something slightly bad (−0.50 €).
We tested this idea in the individual value-coding multivoxel patterns as defined above, using the same individual peak-accuracy searchlights (radius: three voxels) in a 12-mm sphere surrounding the group peak voxel in the OFC and left and right IPS. (Again, changing the size of this search sphere to 8–14 mm led to qualitatively similar results.) In particular, we trained an SVR on multivoxel activity patterns from cues with positive values (5.00 € > 0.50 €) and tested it on activity patterns from cues with negative value (−0.50 € > −5.00 €) (Fig. 6A), and vice versa (results represent the average). This cross-valence value decoding was significant in the OFC (one sample t test, t29 = 2.45, P = 0.02), indicating that differences in values above and below zero entail similar multivoxel ensemble codes. In other words, neural value representations in the OFC are invariant to the valence of the expected outcome. By contrast, this invariance was not observed in the bilateral IPS (left IPS, t29 = −1.23, P = 0.23; right IPS, t29 = 1.31, P = 0.20) (Fig. 6A) which is likely to be caused by the simultaneous presence of value and salience signals in this region (see Fig. 4, Fig. S2, and SI Results for analyses ruling out alternative explanations).
Fig. 6.
Common value and salience scales for appetitive and aversive cues. (A) (Upper) The decoding analysis. SVR models were trained on the value of all appetitive cues and tested on the value of all aversive cues (and vice versa). (Lower) Bars reflect average accuracy (Fisher’s z-transformed correlation) in the OFC and left and right IPS (superior PPC). Accuracies differ significantly between regions (one-way ANOVA; F2,58 = 3.41, P = 0.04). (B) (Upper) The decoding analysis. SVR models were trained on the salience of all appetitive cues and tested on the salience of all aversive cues (and vice versa). (Lower) Bars reflect average accuracy in the ACC and left and right TPJ (inferior PPC). Accuracies do not differ between regions (one-way ANOVA; F2,58 = 0.24, P = 0.79). Error bars depict SEM for n = 30. Asterisks indicate significant accuracy at P < 0.05 (one-sample t test); n.s. indicates nonsignificance at this threshold (i.e., P > 0.05).
We also performed the corresponding analysis for salience in salience-coding regions (ACC and left and right TPJ) to test whether salience encoding for appetitive and aversive cues is similar. We trained an SVR on activity patterns from appetitive cues (5.00 € > 0.50 €) and tested it on activity patterns from aversive cues (−5.00 € > −0.50 €), and vice versa. We find significant cross-valence salience encoding in all three brain regions (one-sample t tests, all Ps < 0.001) (Fig. 6B) and no main effect of region (one-way ANOVA; F2,58 = 0.24, P = 0.79). This result shows that high vs. low appetitive and aversive values are represented by similar activity patterns in all salience-coding regions.
Discussion
Representations of Value and Salience in the PPC.
In the current experiment, we have shown that multivoxel activity patterns in the PPC correlate with both value and salience. Value signals in the PPC have long been investigated in primates (6–9). For instance, in monkeys engaged in saccadic decisions, the activity of LIP neurons scales with the expected reward associated with saccadic targets in the neurons’ response fields (6). Moreover, even if the cue in the response field does not provide action information, these neurons change their activity according to whether the cue predicts reward (39). Our results further reinforce the notion of value coding in the PPC by revealing such signals even in a noninstrumental task.
However, the view that LIP neurons encode value has been challenged recently by a study that used both appetitive and aversive decision outcomes (37). The authors showed that single LIP neurons fire strongly to both highly appetitive and highly aversive outcomes. This firing profile is incompatible with a value account but suggests that LIP neurons actually are coding for the salience of the option in their response fields. Thus, instead of containing information about the value and the particular type of response (approach vs. avoidance), these results suggest that the LIP is signaling the importance of a cue independent of its valence. By using an unbiased whole-brain approach, we reconcile these seemingly contradictory findings and demonstrate that both value and salience signals are present in the PPC. We show that, in line with previous results, the inferior PPC including the TPJ encodes salience (40), whereas the superior PPC primarily encodes value but also shows a trend for salience. Thus, our results suggest that the PPC encodes the importance of cues (37) and is involved not only in shifting attention and accumulating further information (41) but also in guiding utility-maximizing behavior (6). Thus, there are indeed value signals in the PPC, and the conclusions of the previous studies reporting value-coding neurons (6–9) were essentially correct. However, because the designs of these studies did not include aversive stimuli, they may have misclassified salience-coding neurons as value-coding neurons and therefore overestimated the prevalence of value-coding neurons.
Furthermore, our results suggest that, instead of restricting investigations to the LIP, a more comprehensive coverage of additional PPC regions with neurophysiological methods assessing both appetitive and aversive stimuli may be warranted. However, an important issue to consider when comparing neuroimaging with neurophysiology is the lack of correspondence between blood oxygen level-dependent (BOLD) signals and neural spiking. BOLD signals more closely follow input into and local processing within a region rather than the spiking output (42, 43). Thus, because salience signals can be constructed from value input (but not vice versa), it is possible that the value information we observe in the PPC may reflect input or local processing that is not represented in the spiking output of this region. Moreover, the spatial resolution of neuroimaging is limited compared with neurophysiology, and it is conceivable that intercalated populations of value and salience coding neurons could be detected with electrodes but not with scanners. However, even though the link between multivoxel patterns and neurophysiology is not fully understood (SI Discussion), pattern-based analyses revealed results not obtained using univariate analyses (SI Results).
Finally, value signals in the LIP have been questioned based on the finding that phasic, cue-locked signals are related to salience rather than value (37). However, this report was controversial because delay-period activity, which is a classic marker of value- or intention-related activity in the LIP (6–9), was not seen in their data (44). It is possible that the authors recorded from a region different from that used in previous studies and that value and salience signals coexist in the PPC, as suggested by our current findings and a recent inactivation study (45).
Representations of Value in the OFC.
We find value is represented in the central OFC (see SI Discussion on localization). This finding is in line with a large number of animal recording (10–18) and human imaging studies (20–36). Because we used a noninstrumental task with both appetitive and aversive outcomes, our results extend and inform these findings in several ways. First we show that the OFC represents value even in the absence of decisions, that is, independent of action values, chosen values, and other choice signals. Second, by using both appetitive and aversive predicted outcomes, we demonstrate that these anticipatory signals indeed code for value rather than salience.
Moreover, using pattern-based analysis allowed us to show that the positive value of appetitive cues is represented on the same neural scale as the negative value of aversive cues. Specifically, the difference between two expected outcomes, one of which is better (i.e., more desirable) than the other, is represented by the same multivoxel pattern, independent of whether the two outcomes are appetitive or aversive. These results were achieved by training and testing within-subject multivariate models on appetitive and aversive values, respectively. By doing so, we explicitly tested for similar neural codes of appetitive and aversive values and demonstrated that appetitive and aversive values indeed are encoded by the same multivoxel response pattern. Importantly, this finding suggests the presence of neurons that encode appetitive and aversive outcomes on a common value scale (i.e., neurons that consistently change their activity with increasing reward and decreasing punishment). Indeed, individual neurons showing such consistent activity changes with the value of both rewarding and punishing outcomes have been identified in a very similar region of the central OFC in monkeys (14).
Definitions of Salience.
In our experiment, cues deterministically predicted the appetitive and aversive outcomes of different magnitude, and salience was defined as the absolute (unsigned) value (i.e., magnitude) of the outcomes (4, 5, 40). Note, however, that salience may be defined not only through magnitude but also through probability (1–3). In these views, particularly reliable (P = 0, P = 1) predictors of reward have different levels of salience than unreliable (P = 0.5) predictors (2). Regardless of the particular definition, the salience of a cue determines the amount of attention that is recruited for further processing and learning (2, 46). Unfortunately, direct comparisons between probability- and magnitude-based salience concepts are lacking, but here, as in previous experiments (40, 47), we show that magnitude-related increases in attention are reflected in faster responding for more salient cues.
In contrast to our findings, previous research showed probability-based uncertainty (48) and probability-based salience signals in the OFC (47) despite the similar effects on behavior. Probability-based salience was encoded by overlapping neuronal populations with opposing coding schemes, and although they might have been missed in standard univariate BOLD analyses, our pattern-based approach should, in principle, be sensitive to such responses (SI Discussion) (49, 50). Even though we cannot draw firm conclusions from these negative results, we believe that this discrepancy is likely to result from differences in how salience was defined (probability vs. magnitude) rather than from methodological differences (single-cell vs. BOLD responses).
Conclusion.
In summary, here we used cues predictive of appetitive and aversive outcomes and showed that the PPC encodes their value and salience in superior and inferior compartments, respectively. The co-occurrence of value and salience signals in the PPC mitigates discrepancies between previous single-cell recording experiments. Moreover, we have shown that the OFC encodes appetitive and aversive values and represents both values on a common neural scale. Such a common scale is of fundamental importance for economic decision-making because it enables computations across the entire range of possible values.
Materials and Methods
Subjects.
Thirty right-handed subjects (15 male; 24 ± 0.59 y old, mean ± SEM) with normal or corrected-to-normal vision participated in the experiment. The study was approved by the local ethics review board of the Humboldt University of Berlin, and subjects provided informed consent to participate.
Stimuli and Task.
To study neural representations of value and salience, we used a noninstrumental outcome-prediction task (Fig. 1A) in which appetitive and aversive outcomes (gains and losses of 0.50 € and 5.00 €) were deterministically (i.e., with 100% cue−outcome contingency) predicted by two sets of visual cues, resulting in a total of eight cues (Fig. 1B). Associations between cues and outcomes were randomized across subjects. In each trial of the task, one of the eight cues was shown for 2 s. After a variable interval (4–8 s), subjects had to indicate the outcome that is predicted by the cue by pressing one of four buttons (using the left middle, left index, right index, or right middle finger) corresponding to the position of the correct outcome on a response-mapping screen (RMS). Importantly, to prevent preparatory motor signals during the cue interval, the positions of the four outcomes on the RMS were randomized in each trial. After the rating, the outcome was shown to the subject for 2 s. In each of the six scanning runs, each of the eight cue–outcome pairings was shown five times, resulting in 40 trials per run. Subjects received 25 € for participation and were informed that at the end of the experiment they would randomly pick one trial (by throwing a die to select the run, and by drawing the trial number from an urn), and the outcome of the selected trial would be added (appetitive outcomes) or subtracted (aversive outcomes) from their total payment. Thus, each trial outcome had the same probability of being realized.
Before scanning, subjects performed several training sessions. First, they were familiarized with the RMS. Second, subjects performed a classical conditioning session to learn the associations between the eight cues and the four outcomes. Finally, they performed a practice version of the actual scanner task outside the magnet. During conditioning, practice, and scanner sessions, all cues (and thus outcomes) were intermixed pseudorandomly. Cues were separated into sets only for analysis purposes (see below). In the last practice session before subjects went into the scanner, average performance was at 88.83% (t29 = 51.55, P < 0.001, one-sample t test against chance = 25%). Also the RT data showed the same pattern as in the scanner. In particular, there were significant and negative effects of value (t29 = −3.56, P = 0.0013) and salience (t29 = −3.37, P = 0.0022), which did not differ significantly (t29 = 0.55, P = 0.59). This result demonstrates that subjects had learned the cue–outcome associations before entering the scanner.
Behavioral Data Analysis.
We estimated the effects of value and salience on RT using single-subject multiple regression models (21). Specifically, we simultaneously regressed trial-by-trial RT against z-standardized regressors of value and salience on the single-subject level. The resulting standardized regression coefficients reflect the independent effects of value and salience on RT (i.e., how much variance in the RT data is explained by value and salience, respectively). Please note that, given their orthogonality, value and salience can affect RT independently. The regression coefficients then were tested individually for significance on the group level by using one-sample t tests. To compare the regression coefficients corresponding to the effects of value and salience on RT, we used a paired-sample t test.
fMRI Data Acquisition and Preprocessing.
fMRI data were acquired on a 3-Tesla Siemens Trio scanner equipped with a 12-channel head coil. In each of the six scanning runs 310 volumes were acquired (TR = 2 s, TE = 25 ms, 35 slices, ascending order, 3 mm thick, 0.75 mm gap, field of view 192 × 192 mm, matrix 64 × 64 yielding an in-plane resolution of 3 × 3 mm). Preprocessing was performed by using SPM8 (Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, U.K.) and included slice-time correction, realignment, and spatial normalization to a standard MNI template (resampling to 3-mm isotropic voxels). Unsmoothed images were used for the multivoxel pattern analysis, whereas spatial smoothing with a Gaussian kernel of 8 mm FWHM was applied before the univariate analysis.
Multivoxel Pattern Analysis.
We used a searchlight decoding approach that allows whole-brain information mapping without potentially biasing voxel selection (51, 52) in combination with linear kernel SVR (53, 54). In a first step, for each subject and each run, a general linear model (GLM) was applied to the preprocessed functional imaging data. The GLM contained eight regressors for the onsets of the eight different cues (two sets of cues predicting −5.00 €, −0.50 €, 0.50 €, or 5.00 €) and four regressors for the onsets of the four different outcomes (−5.00 €, −0.50 €, 0.50 €, or 5.00 €), respectively (all convolved with a canonical hemodynamic response function), as well as six regressors accounting for variance induced by head motion. The voxelwise parameter estimates of the first eight regressors represent the response amplitudes to each of the eight cues in each of the six scanning runs.
In a second step, these parameter estimates were used as input for two SVR decoding analyses involving either the value or the salience of the cues as labels. The SVR was performed by using the LIBSVM implementation (www.csie.ntu.edu.tw/∼cjlin/libsvm/) with a linear kernel and a preselected cost parameter (c) of c = 0.01. It is important to note that we used a linear kernel which is not at risk for misclassifying neural value representations as salience by exploiting the v-shaped relationship between value and salience as a nonlinear SVR (e.g., radial-basis function) would do. In other words, the linear SVR used here, which is trained to identify salience signals, will perform at chance when only value information is present in the data. For each searchlight (all voxels within a radius of three voxels surrounding the central voxel), we performed a sixfold leave-one-out cross-validation procedure. In each fold, training was based on data from cues from set I in five scanning runs (e.g., runs 1–5), and prediction accuracy was obtained in the independent sixth scanning run (run 6) based on cues from set II. This procedure was repeated six times, each time leaving out a different scanning run for training the SVR and testing it on this omitted scanning run. By using different cue sets to train and test the SVR, we ensured that information about value is not confounded by information about the visual features of the cues (29). The prediction accuracy assigned to the central searchlight voxel was defined as the average Fisher’s z-transformed correlation coefficient between the actual labels of the independent test dataset and the labels predicted by the SVR model. Because correlation coefficients are computed based on model predictions in the independent test data, and not on model fits in the training data, this cross-validation procedure is completely insensitive to potential noise fitting (i.e., overfitting) in the training data (55). For each subject this method results in 3D maps of locally distributed information about value or salience, depending on whether value or salience is used as the label.
To identify brain regions in which individual searchlights containing information about value and salience overlapped significantly, we performed group-level analyses (n = 30 subjects) by using voxelwise one-sample t tests on smoothed accuracy maps (6 mm FWHM). We applied a statistical threshold of P < 0.05, corrected for multiple comparisons (PFWE-corr < 0.05). Based on a priori hypotheses regarding encoding of value and salience, correction was performed within the following anatomical regions of interest from the automated anatomical labeling atlas: OFC (superior orbital gyrus, middle orbital gyrus, and inferior orbital gyrus), PPC (superior parietal gyrus, inferior parietal gyrus, supramarginal gyrus, and angular gyrus) and ACC (anterior cingulum). For display purposes, all corrected results are presented at PFWE-corr < 0.05 and Puncorr < 0.001.
Supplementary Material
Acknowledgments
We thank F. Imamoglu for assistance in collecting data, M. Murusidze for recruiting subjects, and S. Hetzer for help with the scanning sequence. This work was supported by Grants PP00P1_128574 and CRSII3_141965 from the Swiss National Science Foundation and Grant 01GQ0411from the Bernstein Computational Neuroscience Program of the German Federal Ministry of Education and Research, by the Swiss National Centre of Competence in Research in Affective Sciences, and by the Neuroscience Center Zurich.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320189111/-/DCSupplemental.
References
- 1.Mackintosh NJ. Theory of attention - variations in associability of stimuli with reinforcement. Psychol Rev. 1975;82(4):276–298. [Google Scholar]
- 2.Esber GR, Haselgrove M. Reconciling the influence of predictiveness and uncertainty on stimulus salience: A model of attention in associative learning. Proc Biol Sci. 2011;278(1718):2553–2561. doi: 10.1098/rspb.2011.0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce JM, Hall G. A model for Pavlovian learning: Variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87(6):532–552. [PubMed] [Google Scholar]
- 4.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: Rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459(7248):837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400(6741):233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- 7.Yang T, Shadlen MN. Probabilistic reasoning by neurons. Nature. 2007;447(7148):1075–1080. doi: 10.1038/nature05852. [DOI] [PubMed] [Google Scholar]
- 8.Sugrue LP, Corrado GS, Newsome WT. Matching behavior and the representation of value in the parietal cortex. Science. 2004;304(5678):1782–1787. doi: 10.1126/science.1094765. [DOI] [PubMed] [Google Scholar]
- 9.Louie K, Glimcher PW. Separating value from choice: Delay discounting activity in the lateral intraparietal area. J Neurosci. 2010;30(16):5498–5507. doi: 10.1523/JNEUROSCI.5742-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1(2):155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- 11.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex. Nature. 1999;398(6729):704–708. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 12.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441(7090):223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padoa-Schioppa C, Assad JA. The representation of economic value in the orbitofrontal cortex is invariant for changes of menu. Nat Neurosci. 2008;11(1):95–102. doi: 10.1038/nn2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison SE, Salzman CD. The convergence of information about rewarding and aversive stimuli in single neurons. J Neurosci. 2009;29(37):11471–11483. doi: 10.1523/JNEUROSCI.1815-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi S, Pinto de Carvalho O, Schultz W. Adaptation of reward sensitivity in orbitofrontal neurons. J Neurosci. 2010;30(2):534–544. doi: 10.1523/JNEUROSCI.4009-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennerley SW, Behrens TE, Wallis JD. Double dissociation of value computations in orbitofrontal and anterior cingulate neurons. Nat Neurosci. 2011;14(12):1581–1589. doi: 10.1038/nn.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallis JD. Cross-species studies of orbitofrontal cortex and value-based decision-making. Nat Neurosci. 2012;15(1):13–19. doi: 10.1038/nn.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luk CH, Wallis JD. Choice coding in frontal cortex during stimulus-guided or action-guided decision-making. J Neurosci. 2013;33(5):1864–1871. doi: 10.1523/JNEUROSCI.4920-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hare TA, Schultz W, Camerer CF, O’Doherty JP, Rangel A. Transformation of stimulus value signals into motor commands during simple choice. Proc Natl Acad Sci USA. 2011;108(44):18120–18125. doi: 10.1073/pnas.1109322108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.FitzGerald TH, Friston KJ, Dolan RJ. Action-specific value signals in reward-related regions of the human brain. J Neurosci. 2012;32(46):16417–23a. doi: 10.1523/JNEUROSCI.3254-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt LT, et al. Mechanisms underlying cortical activity during value-guided choice. Nat Neurosci. 2012;15(3):470–476, S1–S3. doi: 10.1038/nn.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Doherty JP, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4(1):95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- 23.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Shimojo S, O’Doherty JP. Is avoiding an aversive outcome rewarding? Neural substrates of avoidance learning in the human brain. PLoS Biol. 2006;4(8):e233. doi: 10.1371/journal.pbio.0040233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plassmann H, O’Doherty J, Rangel A. Orbitofrontal cortex encodes willingness to pay in everyday economic transactions. J Neurosci. 2007;27(37):9984–9988. doi: 10.1523/JNEUROSCI.2131-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tom SM, Fox CR, Trepel C, Poldrack RA. The neural basis of loss aversion in decision-making under risk. Science. 2007;315(5811):515–518. doi: 10.1126/science.1134239. [DOI] [PubMed] [Google Scholar]
- 27.Hare TA, O’Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J Neurosci. 2008;28(22):5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FitzGerald TH, Seymour B, Dolan RJ. The role of human orbitofrontal cortex in value comparison for incommensurable objects. J Neurosci. 2009;29(26):8388–8395. doi: 10.1523/JNEUROSCI.0717-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kahnt T, Heinzle J, Park SQ, Haynes JD. The neural code of reward anticipation in human orbitofrontal cortex. Proc Natl Acad Sci USA. 2010;107(13):6010–6015. doi: 10.1073/pnas.0912838107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plassmann H, O’Doherty JP, Rangel A. Appetitive and aversive goal values are encoded in the medial orbitofrontal cortex at the time of decision making. J Neurosci. 2010;30(32):10799–10808. doi: 10.1523/JNEUROSCI.0788-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kahnt T, Heinzle J, Park SQ, Haynes JD. Decoding the formation of reward predictions across learning. J Neurosci. 2011;31(41):14624–14630. doi: 10.1523/JNEUROSCI.3412-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park SQ, Kahnt T, Rieskamp J, Heekeren HR. Neurobiology of value integration: When value impacts valuation. J Neurosci. 2011;31(25):9307–9314. doi: 10.1523/JNEUROSCI.4973-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Martino B, Fleming SM, Garrett N, Dolan RJ. Confidence in value-based choice. Nat Neurosci. 2013;16(1):105–110. doi: 10.1038/nn.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein-Flügge MC, Barron HC, Brodersen KH, Dolan RJ, Behrens TE. Segregated encoding of reward-identity and stimulus-reward associations in human orbitofrontal cortex. J Neurosci. 2013;33(7):3202–3211. doi: 10.1523/JNEUROSCI.2532-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McNamee D, Rangel A, O’Doherty JP. Category-dependent and category-independent goal-value codes in human ventromedial prefrontal cortex. Nat Neurosci. 2013;16(4):479–485. doi: 10.1038/nn.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lebreton M, Jorge S, Michel V, Thirion B, Pessiglione M. An automatic valuation system in the human brain: Evidence from functional neuroimaging. Neuron. 2009;64(3):431–439. doi: 10.1016/j.neuron.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 37.Leathers ML, Olson CR. In monkeys making value-based decisions, LIP neurons encode cue salience and not action value. Science. 2012;338(6103):132–135. doi: 10.1126/science.1226405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wacker P, Deneffe D. Eliciting von Neumann-Morgenstern utilities when probabilities are distorted or unknown. Manage Sci. 1996;42(8):1131–1150. [Google Scholar]
- 39.Peck CJ, Jangraw DC, Suzuki M, Efem R, Gottlieb J. Reward modulates attention independently of action value in posterior parietal cortex. J Neurosci. 2009;29(36):11182–11191. doi: 10.1523/JNEUROSCI.1929-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahnt T, Tobler PN. Salience signals in the right temporoparietal junction facilitate value-based decisions. J Neurosci. 2013;33(3):863–869. doi: 10.1523/JNEUROSCI.3531-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gottlieb J. Attention, learning, and the value of information. Neuron. 2012;76(2):281–295. doi: 10.1016/j.neuron.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 43.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 44.Newsome WT, Glimcher PW, Gottlieb J, Lee D, Platt ML. Comment on “In monkeys making value-based decisions, LIP neurons encode cue salience and not action value”. Science. 2013;340(6131):430. doi: 10.1126/science.1233214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu Y, Yttri EA, Snyder LH. Intention and attention: Different functional roles for LIPd and LIPv. Nat Neurosci. 2010;13(4):495–500. doi: 10.1038/nn.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell CJ, Le Pelley ME. Attention and associative Learning: From Brain to Behaviour. Oxford, UK: Oxford Univ Press; 2010. [Google Scholar]
- 47.Ogawa M, et al. Risk-responsive orbitofrontal neurons track acquired salience. Neuron. 2013;77(2):251–258. doi: 10.1016/j.neuron.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Neill M, Schultz W. Coding of reward risk by orbitofrontal neurons is mostly distinct from coding of reward value. Neuron. 2010;68(4):789–800. doi: 10.1016/j.neuron.2010.09.031. [DOI] [PubMed] [Google Scholar]
- 49.Haynes JD, Rees G. Predicting the orientation of invisible stimuli from activity in human primary visual cortex. Nat Neurosci. 2005;8(5):686–691. doi: 10.1038/nn1445. [DOI] [PubMed] [Google Scholar]
- 50.Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat Neurosci. 2005;8(5):679–685. doi: 10.1038/nn1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haynes JD, et al. Reading hidden intentions in the human brain. Curr Biol. 2007;17(4):323–328. doi: 10.1016/j.cub.2006.11.072. [DOI] [PubMed] [Google Scholar]
- 52.Kriegeskorte N, Goebel R, Bandettini P. Information-based functional brain mapping. Proc Natl Acad Sci USA. 2006;103(10):3863–3868. doi: 10.1073/pnas.0600244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kahnt T, Grueschow M, Speck O, Haynes JD. Perceptual learning and decision-making in human medial frontal cortex. Neuron. 2011;70(3):549–559. doi: 10.1016/j.neuron.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 54.Kahnt T, Heinzle J, Park SQ, Haynes JD. Decoding different roles for vmPFC and dlPFC in multi-attribute decision making. Neuroimage. 2011;56(2):709–715. doi: 10.1016/j.neuroimage.2010.05.058. [DOI] [PubMed] [Google Scholar]
- 55.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: The dangers of double dipping. Nat Neurosci. 2009;12(5):535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.