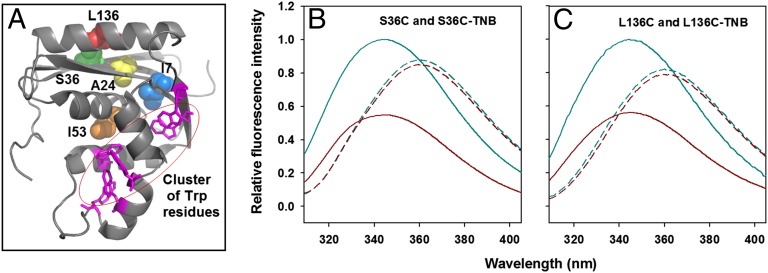
Fig. 1.
TNB quenches the fluorescence of Trp in a distance-dependent manner. (A) Structure of RNase H. The location of I7, A24, S36, I53, and L136 that were replaced by cysteine is shown along with the cluster of Trp residues. The structure was drawn from PDB file 2RN2 by using the program PyMOL (www.pymol.org). Residues 7, 24, and 53 are buried in the native protein and were used for thiol-labeling experiments. Residues 36 and 136 are on the surface of the protein and were used for FRET experiments. S36 and L136 were mutated independently to Cys, to yield two different single-cysteine-containing mutant proteins, S36C and L136C, respectively. The sole thiol moiety in each of the two proteins was labeled with TNB that quenches the fluorescence of Trp in a distance-dependent manner. The TNB-labeled proteins are named as S36C-TNB and L136C-TNB. (B and C) Fluorescence emission spectra of unlabeled and TNB-labeled proteins. In both B and C, the solid cyan line and the solid dark red line denote the fluorescence spectra of unlabeled and TNB-labeled native proteins, respectively. The dashed cyan line and the dashed dark red line denote the fluorescence spectra of unlabeled and TNB-labeled unfolded proteins, respectively. All of the fluorescence spectra in B and C were collected in an identical manner, with the excitation wavelength set to 295 nm.