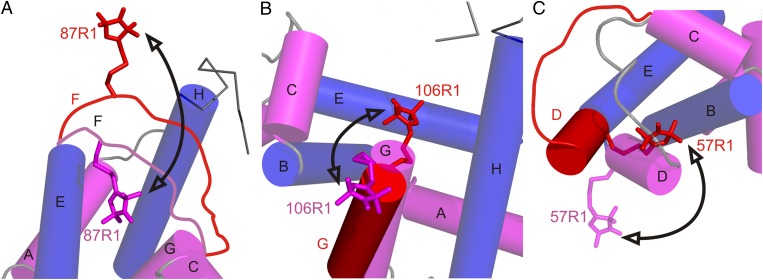
Fig. 5.
Models for the conformational changes of selected sequences in the transition to the pressure-populated MG of apoMb at 2 kbar, pH 6. Shown are the proposed changes relative to the native structure in particular regions. In each panel, the model of the native state is based on the crystal structure of holoMb (PDB ID code: 2MBW), except for the contiguous sequence of the F helix and N terminus of G, which is locally unfolded in the native state of apoMb and is drawn in as a disordered loop in A. This sequence is omitted from B for clarity (66). Helices are shown in cylinder representation; helices that contain reference sites are colored blue, and helices that contain sites that undergo large-amplitude motions in the pressure-populated MG are in magenta. Models for a dominant conformation in the pressure-populated MG based on DEER data at 2 kbar are superimposed and shown in red. Arrows indicate a trajectory of motion followed by each segment in the pressure-populated native-to-MG transition. (A) The sequence of helix F moves out of the heme pocket. The native-state position of the F helix is modeled to satisfy the most probable distance (20 Å) of 31R1/87R1 in apoMb at 0 bar. (B) Helix G fluctuates between the native state and a twist/tilt which inserts R1 into the heme pocket; alternatively, additional fraying of the N-terminal end of helix G at high pressure could result in the disordered sequence containing 106R1 rearranging in a number of distinct conformations that insert the nitroxide into the heme pocket. (C) Helix D fuses with the E helix in a motion that involves a concurrent rotation placing the 57R1 side chain near helix B.