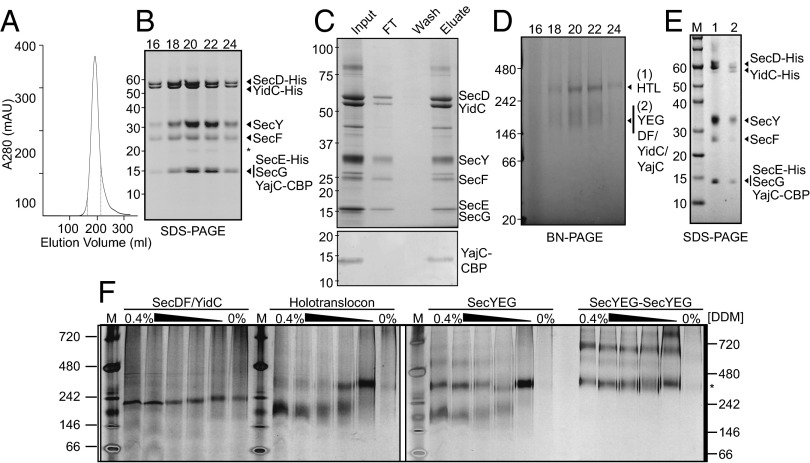
Fig. 1.
Purification of the E. coli HTL. (A) Representative gel filtration/ion exchange elution profile of the HTL (SI Materials and Methods) showing a single peak, demonstrating the copurification of all HTL components in a single complex. (B) SDS/PAGE representation of peak fractions 16–24 (corresponding to elution volumes 165–215 mL of the profile displayed in A, dotted lines). The asterisk represents a proteolytic fragment of SecY. (C) Coomassie-stained SDS/PAGE demonstrating that the purified complex (input) can be reisolated by running the HTL over a calmodulin column (Upper). Detection of the CBP peptide shows that YajC is indeed present in this complex (Lower). (D) BN-PAGE representation of peak fractions 16–24 from B. (E) Second-dimension SDS/PAGE of gel slices taken from the higher-MW (lane 1) and lower-MW (lane 2) complexes seen in D. Note that the slight shift in MW for all components of the HTL is the result of a gel slice being placed into the wells for the run. (F) BN-PAGE illustrating the susceptibility of various complexes [SecDF–YidC, HTL, SecYEG, or a covalently-linked dimer of SecYEG (38)] to changes in detergent concentration. Fifty nanograms of complexes purified in low concentrations of detergent (0.02–0.05%) were resuspended in TSG130 buffer containing decreasing concentrations of DDM from 0.4 to 0%. The asterisk represents migration of the SecYEG tandem dimer.