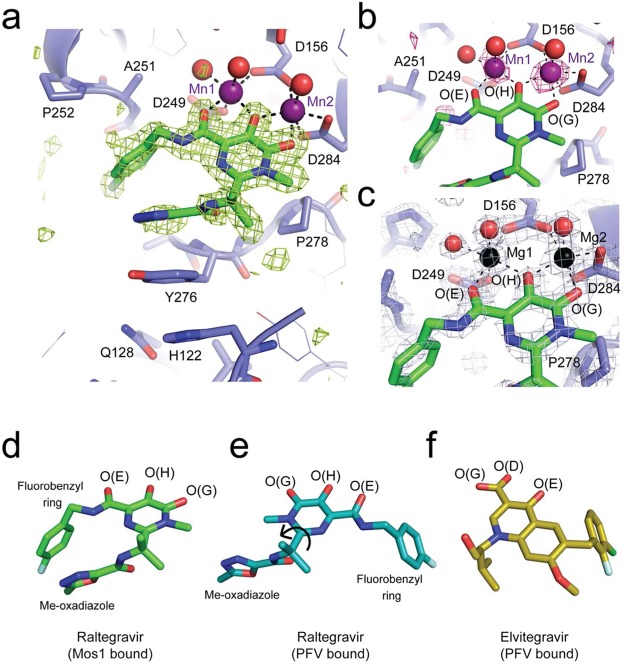
Figure 3.
X-ray crystal structures of Raltegravir bound to the Mos1 transposase catalytic domain. (a) Active site of Mos1 transposase (blue) with Raltegravir (green sticks) bound to Mn2+ ions (purple spheres). The omit map of electron density (green mesh) is contoured at 3σ. Water molecules coordinated to the metal ions are displayed as red spheres. (b) Octahedral coordination of manganese ions in the active site, with anomalous difference electron density map contoured at 2.5σ (pink mesh). (c) Active site of the magnesium ion bound structure, with magnesium ions shown as black spheres. Conformations of Raltegravir bound to (d) Mos1 transposase catalytic domain and (e) the PFV intasome (PDB ID: 3OYA). Rotation about the CBC–CBF bond (indicated by a black arrow) would interconvert these two conformations. (f) Conformation of Elvitegravir bound to the PFV intasome (PDB ID: 3LSU).