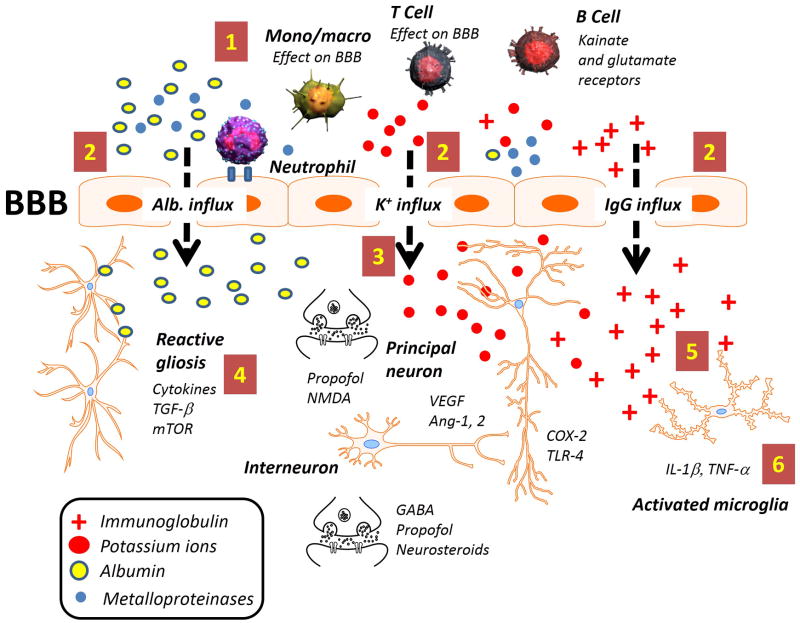
Figure 1. Schematic representation of the immunological players involved in seizure disorders.
Coexistence of central and peripheral inflammatory mechanisms which are potentially epileptogenic requires numerous checkpoints to ensure that infectious or other pro-inflammatory signals are fully activated only under extreme conditions. In this scenario, electrophysiological control of seizure threshold (e.g., endowment of neuronal ion channels) interacts with ictogenic alterations of the brain milieu (soluble inflammatory factors). The latter either directly (potassium ions) or indirectly (albumin) affect neuronal firing. CNS levels of these factors are ultimately controlled by the blood-brain barrier. The most commonly reported excitability changes occur when potassium or glutamate homeostasis is altered [7]. A typical downstream event of inflammation (increased vascular permeability – dotted arrow) will not only alter immediate gene expression or cause sudden excitability changes but also sustain gliosis and activation of microglia [3]. Both principal neurons and interneurons are prone to electrophysiological changes facilitated by pro-inflammatory signals (e.g., COX-2 and IL-1β) or by an abnormal angiogenesis (Ang-2, VEGF). Other brain cells (astrocyte and microglia) contribute to delay recovery from pathologic interstitial homeostasis (e.g., albumin and IgG extravasation). Please see Table 1 for a summary of the molecular players involved.