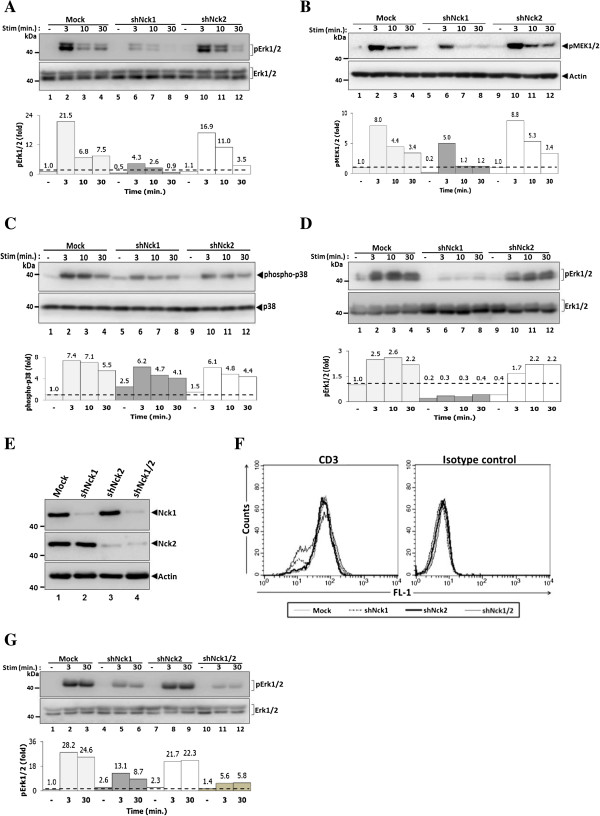
Figure 2.
Nck1, but not Nck2, is essential for activation of Erk1/2 and MEK1/2 proteins. Cells from each monoclonal group were untreated or treated with soluble CD3 antibody (1 μg/ml) at various time points. Cell lysates were subjected to immunoblot with anti-phospho-Erk1/2 antibody (A). Below, the quantified signal intensity of the pErk1/2 was normalized to its total kinase and this value was relative to that in the unstimulated control cells (Mock), set as 1 (black dashed line) and plotted in bar graph. B) Immunoblot analysis of phospho-MEK1/2 from the lysates of cells that were stimulated as in A. Below, signal intensity was quantified and presented as a ratio of p-MEK1/2 to actin relative to that in unstimulated control cells (Mock), set as 1 (black dashed line). C) Immunoblot analysis of phospho-p38. Below, signal intensity was quantified and presented as described in A. D). Polyclonal cells from Mock, Nck1- and Nck2-knockdown cells were stimulated as in A. Below, signal intensity was quantified and presented as described in A. E) Both Nck1 and Nck2 genes were co-silenced in Jurkat T cells. Nck expression levels were analysed by immunoblotting using indicated antibodies. F) The expression levels of surface CD3 molecules on double knockdown Nck1/2 were measured by flow cytometry. G) Cells from each monoclonal group, Nck1-, Nck2- and Nck1/2-knockdown cells were treated as in A for 0, 3 and 30 min. Lysates were subjected to immunoblot with anti-phospho-Erk1/2 antibody. Below, signal intensity was quantified and presented as described in A. Data are representative of at least two independent experiments.