Figure 3. Mechanisms of proteolytic regulation of extracellular VEGF.
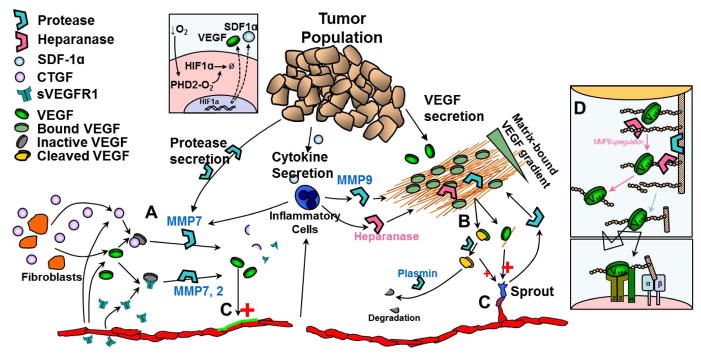
Proteases play a key role in determining the fate of VEGF and its detection by endothelial cells. Proteases can degrade soluble VEGF inhibitors (e.g. CTGF, sVEGFR1) (A) or release matrix-sequestered VEGF (B); both allow free VEGF to escape inactive states and bind to endothelial cell receptors (C). VEGF gradients are altered by release of matrix-sequestered VEGF. In tumor angiogenesis, cancer cells, endothelial cells and inflammatory cells can all contribute to proteolytic activity. VEGF164 has two heparin-binding domains; cleavage of either domain results in a VEGF164/113 intermediate (B), with lower overall affinity for the ECM. Subsequent cleavage of the second domain results in freely diffusing VEGF113. Some MMPs can cleave VEGF bound to heparin/HSPGs (e.g. MMP3) while others cannot (e.g. MMP9). Heparanase activity on cell-surface HSPGs can lead to upregulation of MMP9 leading to cleavage of both GAG chains and core protein (D) and enhanced signaling at the cell surface.
