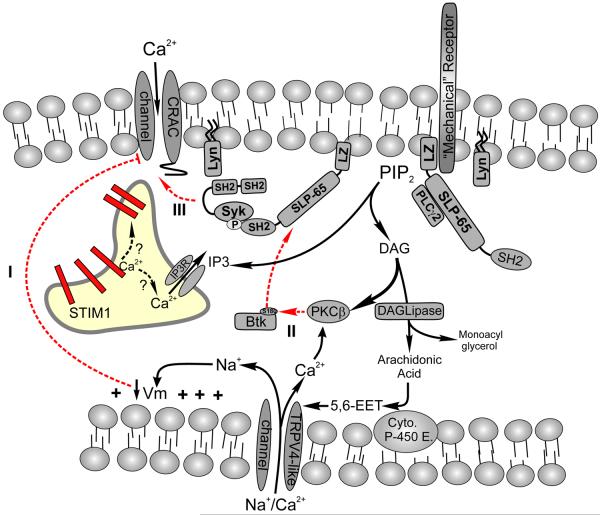
Figure 2. Mechanical regulation of calcium signaling in B lymphocytes.
Shear and osmotic forces mobilize calcium in B lymphocytes via release from intracellular stores and entry across the plasma membrane. The “mechanical receptor” that triggers the calcium signaling cascade in B cells has not been identified yet subsequent steps have been defined. Mechanical and osmotic stress activate PLCγ-2 and the resulting calcium signal is critically dependent on both PLCγ-2 activity and on the expression of the adaptor SLP-65 to which PLCγ-2 anchors. In contrast to BCR-mediated signals, calcium entry in response to mechanical/osmotic stress occurs via a store-independent non-selective cation channel (NSCC) whose pharmacological and biophysical properties most closely resemble those of the transient receptor potential (TRP) channel TRPV4. TRPV4 like channels in B cells are activated by Arachidonic cleaved from DAG which is further metabolized by cytochrome P450 epoxygenase to form 5,6-epoxyeicosatrienoic acid (5,6-EET); a direct channel agonist. Interestingly, although PLCγ-2 is activated and high levels of IP3 are generated by hypotonic stimulation of B cells, CRAC channels do not appear to play any role in the resulting calcium elevations. Several mechanisms could account for the apparent inability to activate CRAC channel mediated calcium entry. Calcium permeation of CRAC channels is highly dependent upon a hyperpolarized membrane potential (Vm). Calcium and sodium permeation of TRPV4-like channels in B cells produces membrane depolarization, which could attenuate Ca2+ permeation of activated CRAC channels (1). Alternatively, because mechanical stimuli produce much higher levels of IP3 than BCR engagement, and therefore DAG, this may activate PKCβ which inhibits Btk membrane localization (2) and this may regulate distal steps in CRAC channel activation following store depletion by IP3 (see text for a more detailed explanation). The mechanism by which Btk potentiates CRAC channel activity is not known.