Abstract
Objective
Oncolytic virotherapy is a promising modality in endometrial cancer (EC) therapy. In this study, we compared the efficacy of the Copenhagen and Wyeth strains of oncolytic vaccinia virus (VV) incorporating the human thyroidal sodium iodide symporter (hNIS) as a reporter gene (VVNIS-C and VVNIS-W) in EC.
Methods
Infectivity of VVNIS-C and VVNIS-W in type I (HEC1A, Ishikawa, KLE, RL95-2, and AN3 CA) and type II (ARK-1, ARK-2, and SPEC-2) human EC cell lines was evaluated. Athymic mice with ARK-2 or AN3 CA xenografts were treated with one intravenous dose of VVNIS-C or VVNIS-W. Tumor regression and in vivo infectivity were monitored via NIS expression using SPECT-CT imaging.
Results
All EC cell lines except KLE were susceptible to infection and killing by VVNIS-C and VVNIS-W in vitro. VVNIS-C had higher infectivity and oncolytic activity than VVNIS-W in all cell lines, most notably in AN3 CA. Intravenous VVNIS-C was more effective at controlling AN3 CA xenograft growth than VVNIS-W, while both VVNIS-C and VVNIS-W ceased tumor growth and induced tumor regression in 100% of mice bearing ARK-2 xenografts.
Conclusion
Overall, VVNIS-C has more potent oncolytic viral activity than VVSIN-W in EC. VV appears to be most active in type II EC. Novel therapies are needed for the highly lethal type II EC histologies and further development of a VV clinical trial in type II EC is warranted.
Introduction
Oncolytic virotherapy is an emerging treatment modality that uses replication-competent viruses to specifically destroy cancers. The potential of oncolytic virotherapy using herpes simplex virus, measles virus, vesicular stomatitis virus, vaccinia virus, adenovirus, retrovirus, poliovirus has been demonstrated by numerous preclinical and clinical studies [1]. Oncolytic viruses are genetically engineered so that they are attenuated, armed and/or retargeted [1]. Poxvirus, particularly vaccinia virus (VV), is a promising oncolytic virus due to its well-characterized safety profile with its extensive use in the World Health Organization’s smallpox eradication campaign during the 1960s [2]. Vaccinia virus has a large genome which can be easily genetically modified and can accommodate inserts exceeding 25 kb using homologous recombination [3]. Also, even though VV is known to infect a wide range of cells, it replicates and propagates productively only in cancer cells [4]. Therefore, placing a reporter or therapeutic gene under a late promoter has the advantage of ensuring high levels of transgene predominantly in the cancer cells.
There are a number of preclinical and clinical studies using the Western Reserve, Lister and Wyeth strains of VV in cancer [1, 5, 6]. A number of VV strains differing in pathogenicity and host range exist mainly due to the evolution of the virus in different parts of the world during smallpox vaccination history [7]. The New York City Board of Health (NYCBH) strain was originally used for smallpox vaccination in the United States. The Wyeth strain has been used extensively as vaccines in clinical trials. The Western Reserve (WR) is a particularly virulent strain derived from Wyeth after passage in laboratory mice. Other strains, such as Copenhagen, Lister, IHD-W, and IHD-J, and several attenuated strains, such as the modified Vaccinia Ankara, are also frequently used for various applications [7]. Several different full length VV genomes have been used for rescue of oncolytic viruses including WR [8], Wyeth[9], Copenhagen[10], and Lister [11].
The human sodium iodide symporter (hNIS) is a membrane-bound glycoprotein present on the basolateral surface of thyroid follicular cells which concentrate iodine required for synthesis of thyroid hormones. NIS expression in thyroid tissue is exploited clinically when radioiodine I123 is used in imaging for thyroid disorders and I131 is used in radiotherapy for thyroid cancers. Ectopic expression of NIS in a cell also enables it to concentrate iodine from its surroundings. Several oncolytic viruses, such as measles virus (MV), vesicular stomatitis virus (VSV), adenovirus (Ad), and herpes simplex virus (HSV) have been engineered to express the NIS gene to enable noninvasive, real-time monitoring of the pharmacokinetics of viral replication in tumors using I123 or I125 with SPECT/CT imaging or to combine with I131 radiotherapy to increase the efficacy of tumor cell killing [12–14]. MV expressing hNIS (MV-NIS) is being evaluated in several phase I clinical trials in patients with ovarian cancer (intraperitoneal administration), multiple myeloma (intravenous), squamous cell carcinoma of the head and neck (intratumor) and mesothelioma (intrapleural) [12, 15, 16].
Endometrial cancer (EC) is the most common gynecologic malignancy in the United States [17] and optimal systemic treatment for advanced stage and recurrent disease remains undefined. Systemic chemotherapy with or without radiation [18, 19], hormone therapy [20], and most recently, biologic agents such as lapatinib [21] and bevacizumab [22] have been and continue to be investigated in metastatic and recurrent EC. Unfortunately, the response rates for most systemic therapies investigated in metastatic EC remain in the single-digit percentages and overall survival continues to decline in this patient population [23, 24]. Novel systemic therapies are desperately needed.
Oncolytic virotherapy appears to be a promising systemic therapy for EC. While there have only been a few women with metastatic EC who have been treated on oncolytic virotherapy clinical trials [25], the potency of more novel oncolytic viruses in EC is encouraging. We have previously shown that both oncolytic MV and VSV treatment result in effective tumor control in 100% of mice bearing EC xenografts [26]. Here we compare in vitro oncolysis and preclinical efficacy in EC of two different strains of oncolytic VV expressing the hNIS transgene. Expression of hNIS and I125 uptake were studied in xenografts treated with either the Copenhagen (VVNIS-C) or Wyeth (VVNIS-W) strains of VV in order to track the sites of viral infection and better understand the mechanism of VV oncolysis.
Materials and Methods
Cells, plasmids, and viruses
The human type I EC cell lines HEC1A, Ishikawa, KLE, RL95-2, and AN3 CA and type II cell lines ARK-1, ARK-2, and SPEC-2 cells were used. KLE and RL95-2 were cultured in Dulbecco Modified Eagle Medium (DMEM) and Ham F-12 Nutrient Mixture (DMEM/F12; Mediatech, Herndon, Virginia) supplemented with 10% FBS (Life Technologies, Grand Island, New York). AN3 CA and Ishikawa were cultured in 10% FBS DMEM (Mediatech). HEC1A, ARK-1, ARK-2, and SPEC-2 were maintained in 10% FBS RPMI-1640 (Mediatech). The monkey kidney (Vero; CCL-81), human osteosarcoma (U2OS), and human cervical cancer (HeLa) cells were purchased from American Type Tissue Collection (ATCC). Vero cells were maintained in 5%FBS DMEM. Both U2OS and HeLa were maintained in 10%FBS DMEM.
Human NIS gene (NM_000453.2) was synthetized by GeneART (Regensburg, Germany). Synthetized hNIS gene was cloned into pSEL-eGFP (a gift from Dr. David Bartlett, University of Pittsburgh) [27] by replacing the xanthine-guanine phosphoribosyl transferase (gpt) gene. Oligos for a late promoter sequence (pSL4) with HindIII and XhoI restriction enzyme sites (AAGCTTACAAAAAAAACTTCTCCAAATAGACTCGAG) were ordered from Invitrogen (Life Technology, Grand Island, NY), annealed and replaced the p7.5 promoter in pSEL-eGFP to form pSC65-eGFP-pSL4-hNIS.
To make the recombinant VVNIS-C and VVNIS-W, U2OS cells were infected with either wild Wyeth strain or Copenhagan strains of vaccinia viruses at a multiplicity of infection (MOI) of 0.01 and then transfected with pSC65-eGFP-pSL4-hNIS using Lipofectamine 2000 (Invitrogen). Cells were incubated at 37°C for 4 hours, the medium was replaced, and then cultured for two more days. Viruses were released from the cell debris via 3 freeze-and-thaw cycles. Harvested viruses were used to infect freshly prepared U2OS cells overnight. GFP-positive cells were sorted using flow cytometry and purified by selecting 3 more rounds of GFP-positive plaques on U2OS cells with CMC overlay [3]. As a final step of plaque purification, viruses from the selected plaque were filter into a U2OS plate through a 0.65 µM filter (Sartorius Stedim, Goettingen, Germany) and agarose overlay was applied instead of CMC overlay. The resultant viruses, VVNIS-C and VVNIS-W, were further amplified in HeLa cells for in vitro and in vivo experiments.
Virus Infection, Cell Viability, and Progeny Production
For the virus killing and cell viability assays, cells in 96 well plates were exposed to either the VVNIS-W or VVNIS-C at specified multiplicity of infection (MOI; 0, 0.001, 0.01, 0.1, 1, and 10). Cell viability was assessed at 72 hours post infection using the (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell proliferation assay according to manufacturer’s instructions (Promega, Madison, WI). Photos were taken using a Nikon Eclipse TE300 microscope mounted with a Nikon U2 digital camera prior to the MTS assay. The median effective concentration (EC50) values of the viruses in each cell line were calculated using PRISM analysis software (GraphPad, La Jolla, CA).
For the viral progeny propagation assays, cells (2×105/well) were seeded in 12 well plates, infected with viruses (MOI 0.02) and incubated at 37°C. Two hours later, the virus inoculum was removed and replaced with growth media. Cells were harvested at 48 hours post virus infection, and viral titers were determined by TCID50 assay on HeLa cells.
Flow Cytometry
Cells (6×105/well) in 6 well plates were infected with virus, VVNIS-W or VVNIS-C, at MOI 0.1 in 1 mL serum-free OPTI-MEM. After 2 hours, the virus medium was removed, replaced with 1 mL growth medium and incubated at 37°C. Cells were collected at 24 hours post infection with VV, washed with PBS, and fixed in 500uL 4% Paraformaldehyde for 90 minutes. The cells were washed and re-suspended in 500 uL PBS. The percent of GFP positive cell was measured by flow cytometry (BD BioSciences, FACScan) and the data was analyzed using FlowJo software (Tree Star, Ashland, OR).
I125 uptake in vitro
Cells (2×105/well) in 12 well plates were infected with viruses (MOI 0.1) and incubated at 37°C. 24 hours after infection, cells were washed with Hank’s Balanced Salt Solution (HBSS; VWR, Radnor, PA) and replaced with HBSS supplemented with 10mM of HEPES. 125I (500,000cpm) was then added into each well with or without KClO4 to inhibit NIS mediated uptake of 125I and incubated at 37°C. 2 hours after incubation, cells were washed with iced HEPES-HBSS 3 times and replaced with 1M NaOH (500ul/well). The NaOH solution was then transferred to polypropylene tubes 10 minutes later and the radioactivity was quantitated using Isodata-10 gamma counter (ICN Biomedicals, Costa Mesa, CA).
Animal Experiments
All animal experiments were approved by and performed according to the guidelines of the Mayo Clinic Institutional Animal Care and Use Committee. 4- to 5-week-old female athymic mice were purchased from Harlan (Indianapolis, Indiana). To study the effect of different viruses on tumor growth and animal survival, mice were implanted subcutaneously in the right flank with 2×106 ARK-2 or AN3 CA cells. When tumors reached 0.3 to 0.5 cm in diameter, 100 µL of viruses or PBS (vehicle control) was injected intravenously through the tail vein (106 TCID50/mouse). Tumors were measured twice to three times per week, and mice were euthanized when tumor mass reached 10% of the mouse’s body weight, became ulcerated or interfered with a mouse’s ability to reach food or water.
During the efficacy study, NIS gene expression was also monitored using a SPECT-CT imaging machine (U-SPECT-II, MI Labs, Netherlands) at day 7, 14, 21 post treatment. At the day of imaging, two mice from each group were injected with 300 uCi of I125 intraperitoneally 1hour before imaging and then imaged for 20–25 minutes. The DICOM image files were blinded and analyzed by a nuclear imaging service provider (Imanis Life Sciences, Rochester, MN).
Statistical Analysis
The tumor size was compared between the three treatment groups with a mixed-effects model with a random effect for mouse and fixed effects for time and treatment, including a quadratic effect for time as well as the time-by-treatment interaction. All analyses were performed using JMP version 9 software (2010 SAS Institute).
Results
EC cell viability after VVNIS infection in vitro
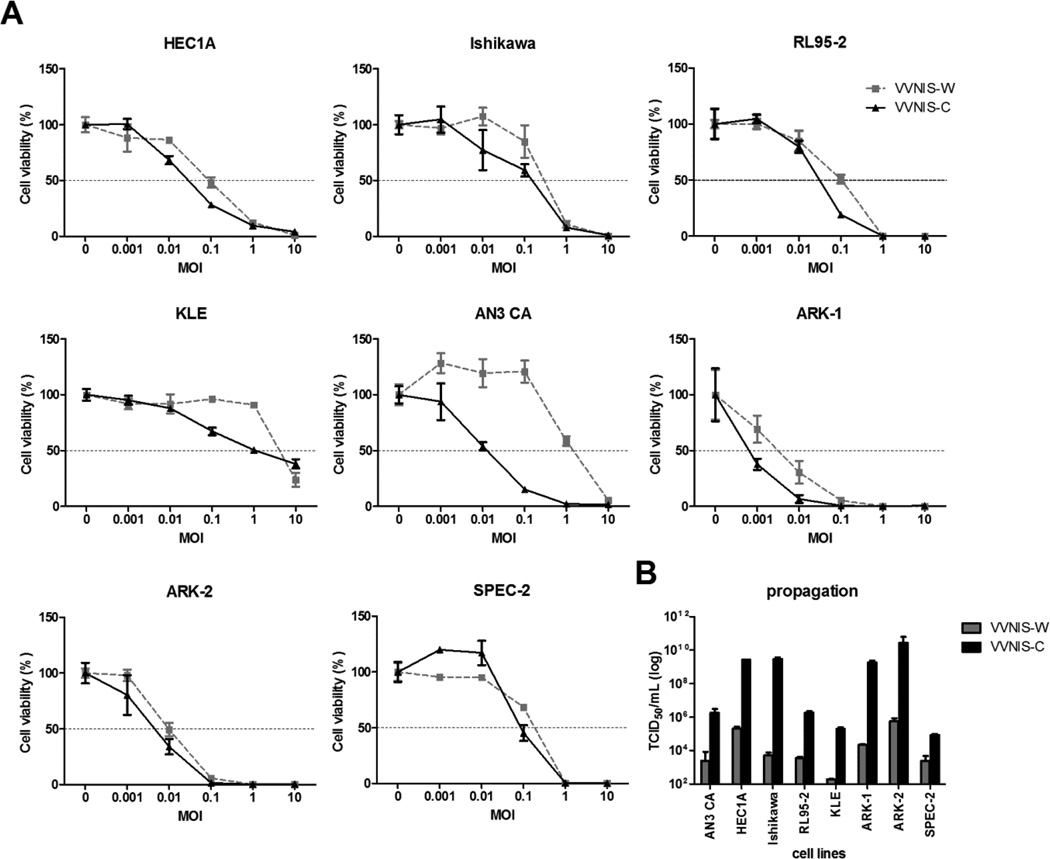
A panel of 8 human endometrial cancer cell lines was used to compare the potency of VVNIS-C and VVNIS-W in vitro at various MOIs. Most of the EC cell lines, except for KLE, were susceptible to infection and killing by both VVNIS-C and VVNIS-W. Type I EC cell lines were less efficiently killed by both VVNIS-C and VVNIS-W when compared to the Type II EC cell lines (Figure 1a and Table 1). By 72 hours, almost 100% of Type II EC cells were killed by both VVNIS at MOI 0.1 or 1, while a MOI of 1 or 10 was required to achieve 100% killing of Type I EC cell lines (Figure 1a). KLE was highly resistant infection by VVNIS-C and VVNIS-W. iOnly 25% of KLE cells were killed at an MOI of 10. Both VVNIS-W and VVNIS-C had comparable potency in all cell lines tested, except in AN3 CA cells where VVNIS-C was substantially more potent (Figure 1a and Table 1). The concentration of VVNIS-C required to kill 50% of AN3 CA cells (EC50) was about 80-fold lower than VVNIS-W (Table 1).
Figure 1.
Virus induced cell killing and viral progeny propagation in human EC cell lines. A, Cell viability of Type I EC cell lines, AN3 CA, Ishikawa, HEC1A, KLE, and RL95-2, and Type II EC cell lines, ARK-1, ARK-2, and SPEC-2, at 72 hours post VV infection. B, Cells were infected with VV at low MOI (0.02) to enable multiple-cycles of infection. The amount of viral progeny was determined by TCID50 assay 48 hours later.
Table 1.
EC50 values of VVNIS-W and VVNIS-C in EC cells 72 hours after infection
| VVNIS-W | VVNIS-C | |
|---|---|---|
| AN3 CA | 0.98 | 0.012 |
| HEC1A | 0.11 | 0.023 |
| Ishikawa | 0.196 | 0.12 |
| RL95-2 | 0.1 | 0.025 |
| ARK-1 | 0.002 | 0.00034 |
| ARK-2 | 0.0099 | 0.0050 |
| SPEC2 | 0.18 | 0.09 |
Viral titers calculated from the TCID50 assay on HeLa cells 48 hours after infection with VVNIS showed that most of the EC cell lines supported both VVNIS-C and VVNIS-W replication (Figure 1b). KLE and SPEC2 showed the lowest output of progeny virus, while higher titers were observed in HEC1A, Ishikawa, ARK-1, and ARK-2. VVNIS-C infection resulted in at least 100-fold higher viral titers than VVNIS-W in all 8 human EC cell lines, suggesting that the Copenhagen strain is more potent in its ability to propagate and spread in the human EC cells than the Wyeth strain.
Early infectivity and spreading of VVNIS in EC in vitro
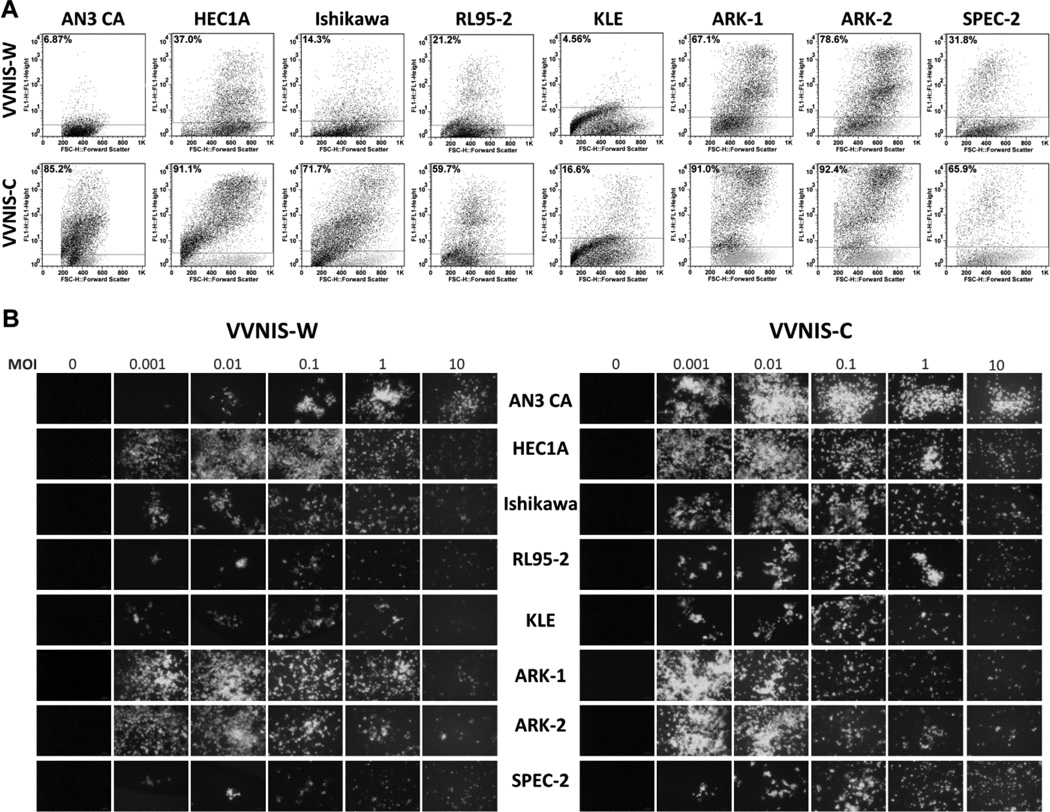
The infectivity of VVNIS-C and VVNIS-W in EC cell lines was determined by measuring the percentage of GFP positive cells at early time points after infection but before significant cell death occurred. Overall, EC cell lines were more susceptible to infection by VVNIS-C than VVNIS-W (Figure 2a). The most impressive difference was seen in AN3 CA cells where 85.2% of cells were infected by VVNIS-C compared to only 6.87% by VVNIS-W. Type II EC cell lines were also highly susceptible to infection by VVNIS-C and VVNIS-W where 67.1% (ARK-1) and 78.6% (ARK-2) of cells were GFP positive at 24 hours after VVNIS-W infection. KLE cells were resistant to infection by both VVNIS-C and VVNIS-W with only 4.6% (VVNIS-W) and 16.6% (VVNIS-C) of cells being GFP positive 24 hours post infection (Figure 2a).
Figure 2.
Infectivity and spread of VVNIS in human EC cell lines. A, Cells were infected with viruses at MOI 0.1 and the percent of GFP positive cells were analyzed by flow cytometry 24 hours after infection. Numbers within each panel indicate the percentage of GFP positive cells. B, Photographs of infected EC cells at 72 hours after infection at various multiplicity of infection.
The ability of the virus to spread within the culture was monitored by GFP fluorescence at low and high MOIs (Figure 2b). By 48 hours, there were abundant GFP-positive cells in the VVNIS-W and VVNIS-C infected cultures despite the low MOI of 0.001 (Figure 2b). For cell lines that were highly susceptible to VVNISs infection (e.g. ARK-1 and ARK-2), the GFP signals decreased due to an increase in cell death with increase in MOI. For cell lines that were more resistant to virus infection (e.g. AN3 CA with VVNIS-W or KLE for both viruses), the GFP signals increased with the increase in MOI (Figure 2b).
I125 uptake in EC cell lines infected with VVNISs in vitro
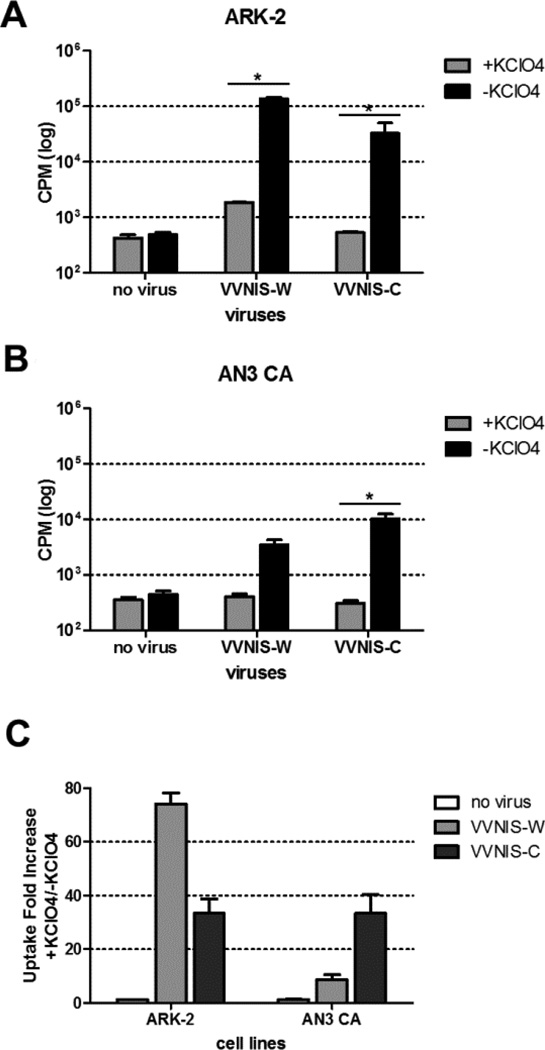
Prior to in vivo experiments to determine the oncolytic activity of VVNIS in EC xenografts, we evaluated the ability of VVNIS-infected cells to concentrate radioiodine in an I125 uptake assay, with and without the addition of potassium perchlorate (a competitive inhibitor of NIS-mediated iodine uptake), in type I (AN3 CA) and type II (ARK-2) cell lines. There was significant I125 uptake in both VVNIS-W and VVNIS-C infected ARK-2 cells at 50–100 fold above that of uninfected cells (Figure3a and 3c). The I125 uptake was specifically due to NIS as potassium perchlorate effectively prevented I125 uptake in these infected cells. In contrast, I125 uptake in VVNIS infected AN3 CA cells were lower compared to ARK- 2. AN3 CA cells were able to concentrate I125 at 10–40 fold above background (Figure3b and 3c). Due to the higher infectivity of VVNIS-C in AN3 CA cells, there was correspondingly higher I125 uptake in the VVNIS-C infected culture compared to VVNIS-W infected culture.
Figure 3.
Assay to measure NIS activity in infected EC cells. (A) ARK-2 and (B) AN3 CA cells were mock infected (no virus) or infected with viruses at MOI 0.1 and uptake of I125 (counts per minute CPM) was analyzed in the presence or absence of KClO4 at 24 hours after infection. C. Fold increase in I125 uptake in VVNIS infected ARK-2 and AN3 CA were calculated by dividing the uptake amount in the absence of KClO4 by the uptake amount in the presence of KClO4. * P<0.05 (Unpaired student t test).
Intravenous VVNIS causes oncolysis in both type I and type II EC xenografts
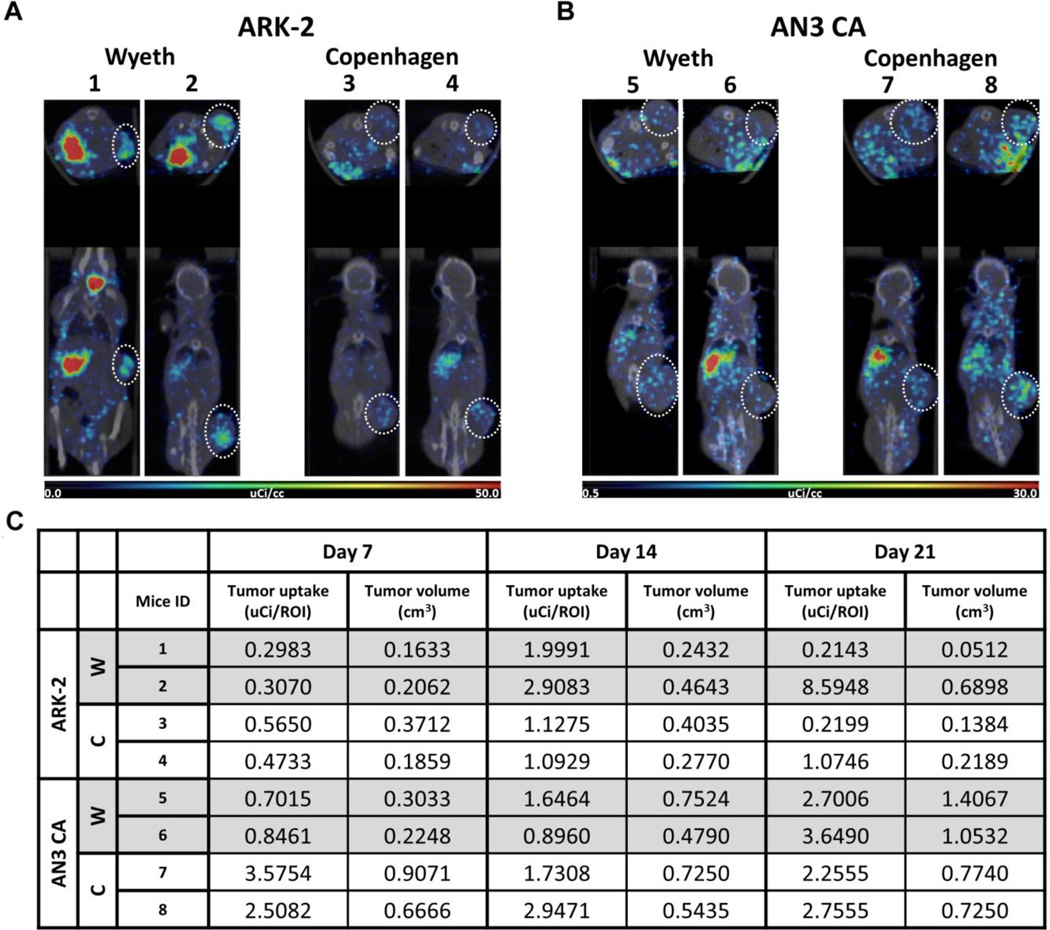
We next compared the oncolytic activity of VVNIS-C and VVNIS-W in ANC 3A (type I EC) and ARK-2 (type II EC) xenografts. When subcutaneous tumor xenografts reached 0.3 to 0.5 cm in diameter, mice were administered one intravenous dose of 106 TCID50 of VVNIS-C or VVNIS-W. A cohort of mice was used for SPECT-CT imaging to track NIS expression in the tumors at day 7, 14, and 21 post-treatment. Figure 4 shows the SPECT-CT images and quantitation of the amount of isotope uptake in tumors of mice at day 14 post-treatment. There was I125 uptake in the thyroid and stomach which are sites of endogenous NIS expression. In addition, strong I125 signals were seen in the tumors of all 8 imaged mice (Figure 4a, b). The I125 uptake signals gradually increased as the virus spread in the enlarging tumors and decreased with decreasing tumor size as the tumors responded to the VV therapy (Figure 4c). I125 uptake was detected in most of the tumors even until day 21 post treatment, suggesting ongoing viral replication in the tumors grown in these immunocompromised mice (Figure 4c).
Figure 4.
Noninvasive SPECT-CT monitoring of the pharmacokinetics of VVNIS infection in EC xenografts. Mice with subcutaneous EC xenografts were injected intravenously with VVNIS-C or VVNIS-W. On the day of imaging, each mouse was receiving 300 µCi I125. A and B, SPECT/CT fused images of mice bearing ARK-2 (A) or AN3 CA (B) xenografts on day 14 post virus infection. Location of tumor on each mouse is circled. C, Tumor I125 uptake (uCi/ROI) and tumor volume (cm3) in each mouse on day 7, 14, 21 post virus infection.
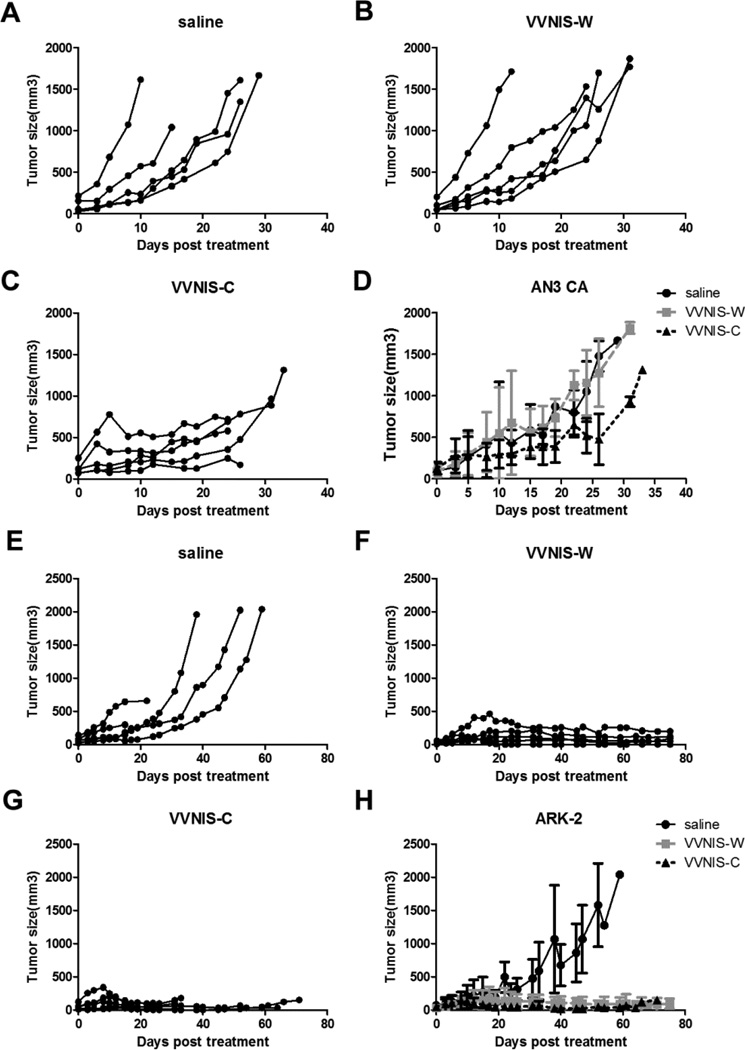
For both xenograft models, tumors in the saline control group continued to grow over time, and mice were euthanized because of tumor burden or tumor ulceration (AN3 CA, Figure 5a; ARK-2, Figure 5e). For the AN3 CA xenograft model, tumors in VVNIS-W treated mice continued to grow over time (Figure 5b) and the effect of time on tumor growth in the VVNIS-W treatment group was not significantly different from the saline control (p value= 0.8401, Figure 5d). In contrast, VVNIS-C treatment attenuated AN3 CA tumor progression (Figure 5c). While there were no complete regressions observed in AN3 CA xenografts treated with VVNIS-C, there was significantly slower growth in those treated with VVNIS-C compared to saline (p value < 0.0001, Figure 5d). For mice bearing ARK-2 xenografts (Figure 5e–5h), therapy with both VVNIS-W (Figure 5f) and VVNIS-C (Figure 5g) effectively halted tumor progression and induced tumor regression in 100% of mice. In fact, 2 of the 10 VVNIS treated mice (1 from VVNIS-W, 1 from VVNIS-C) had complete tumor regression. ARK-2 xenografts treated with either VVNIS-C or VVNIS-W had impressive responses compared to xenografts in the saline cohort (both p value< 0.0001, Figure 5h).
Figure 5.
Tumor response curves post intravenous administration of VVNIS-C or VVNIS-W in mice with subcutaneous (A–D) AN3 CA or (E–H) ARK-2 xenografts. Mice were randomly assigned to a single IV injection of saline, VVNIS-C or VVNIS-W (106 TCID50). A–C and E–G, Individual tumor volumes in each mouse. D and H, Average tumor volumes of each study group.
Discussion
Despite contemporary systemic therapies, the persistently high mortality rate secondary to metastatic and recurrent EC drives the imperative to develop novel therapeutics for this cancer. We have previously shown that VSV as well as the Edmonston strain of MV have potent oncolytic activity against EC both in vitro and in vivo making systemic oncolytic virotherapy an attractive new therapy to study in EC [26]. Prior to finalizing a candidate virus for clinical trial development, we investigated the antitumor activity of VV in the same panel of EC cell lines. Two new recombinant VVs (Copenhagen and Wyeth strains) encoding the human NIS gene were generated to enable noninvasive monitoring of the pharmacokinetics of virus replication. Expression of NIS in the tumors also allowed us to reliably correlate tumor response with real-time virus replication and gene expression in the target lesions. Overall, VVNIS-C appears to have superior oncolytic activity compared to VVNIS-W in EC. Additionally, both type I and type II ECs are susceptible to VV infection and oncolysis. But most notable is the impressive oncolytic activity of VV against type II EC which is particularly recalcitrant to present-day adjuvant therapies.
While type II EC accounts for only 20% of all ECs diagnosed, type II histologies are highly lethal with a median overall survival of only 2 years among stage IV cases treated with curative intent multimodal therapy [28, 29]. Type II EC clinically behaves more aggressively than type I EC with a higher propensity to present at an advanced stage. Type II EC is also particularly refractory to systemic chemotherapy [28] and novel effective systemic agents are vital in the efforts to improve survival from this aggressive form of EC. Both VV and VSV [26] appear to have potent oncolytic activity against type II EC. While MV has activity against EC [26], population-wide MV vaccination precludes systemic MV administration as antibody neutralization of the virus occurs rapidly after intravenous administration [30].
One of the major advantages of VVs over other oncolytic viruses is their potential ability to spread systemically though the blood [8, 9, 27]. VV produces several distinct antigenic forms of viral particle, including an enveloped virus form (EEV) which is capable of evading recognition by complement and neutralizing antibodies by shrouding itself in a host cell-derived envelope that contains several host complement control proteins and few exposed viral proteins [31, 32]. Therefore, the systemic delivery of VVs and their spread between tumors may be highly efficient [33] making it an attractive agent for the treatment of widely metastatic or multi-site recurrent EC.
Interestingly, vaccine strains of VV have already been shown to inherently target cancer [33]. The replication and spreadof VV is associated with activation of the epidermal growth factor receptor (EGFR)-Ras signaling pathway in cancer cells [34]. Thus, in addition to deregulation in cell cycle control and immune evasion that makes cancer cells more susceptible to virus infection, activation of EGFR-Ras pathway in most human cancers suggests that VV could be particularly suited as a selective oncolytic agent [35]. To further enhance the cancer selectivity of VV, a range of viral gene deletions that reduce the ability of the virus to productively replicate in most normal cells have been introduced [36, 37]. In both VVNIS-C and VVNIS-W, the vaccinia encoded thymidine kinase gene (TK) was deleted and replaced by the hNIS gene to further enhance the safety and specificity profiles of the VV [27]. Deletion of the vaccinia encoded TK gene results in dependence of the virus on cellular thymidine kinase which is constitutively expressed at high levels in most cancers but only transiently expressed during the S phase of the cell cycle in proliferating normal cells [36]. Despite deletion of TK in VVNIS-C and VVNIS-W there was toxicity associated with VV virotherapy in our immunocompromised mice, especially for the Copenhagen strain. VV infection of normal tissues resulted in weight loss and pox formation on the tails and feet in some of the VV treated athymic mice. This suggests that further attenuation of the VV might be required, especially if the viruses will be administered to immunocompromised cancer patients.
Alternatively, we also found that among the EC cell lines tested, one cell line (KLE) was particularly resistant to VV infection. Interestingly, this same cell line has previously been shown to be resistant to MV and VSV infection [26]. Some tumor cells are intrinsically resistant to VSV due to constitutive induction or expression of high levels of antiviral interferon (IFN) responsive genes such as OAS and MXA [38]. However, VV encodes multiple genes that can antagonize the host cell antiviral responses [39] and further exploration of the mechanism of resistance to MV, VSV, and VV infection should contribute to improving the therapeutic index of these viruses.
The strains of VV used in different areas of the world during the smallpox eradication program vary in their characteristics, pathogenicity and host range, probably due to variations in the expression or functionality of different virulence genes between strains [7, 33]. Clinical trials where the oncolytic VV from the Wyeth strain, JX594, engineered to express GM-CSF, was administered intratumorally to patients with nonresectable hepatocellular carcinoma led to objective responses in three of ten evaluable patients [1, 37]. Oncolytic VVs from other strains, such as WR strain (vvDD-CDSR, also called JX-929) and Lister strain (GL-ONC1), are also being evaluated in clinical trials against solid tumors, squamous cell carcinoma of the head and neck (SCCHN), and peritoneal carcinomatosis [1, 8, 40], a common presentation of advanced stage type II EC.
In summary, both strains of VV have oncolytic activity against most EC cell lines, although the Copenhagen strain appeared to be more potent with higher rates of infectivity and substantially higher progeny production (10–106 fold) in infected cells compared to the Wyeth strain. In vivo, the Copenhagen strain was effective at controlling tumor growth in both type I and type II EC. Given these findings, we believe that the Copenhagen strain should be pursued further for Phase I testing although the formation of pox lesions in the immunocompromised mice is concerting. To attenuate the virus, we plan to plaque purify the virus further to identify an isolate that has high oncolytic activity but does not cause lesions in the immunocompromised mice before initiating a clinical trial of VVNIS-C in type II EC.
Highlight.
Both Type I and Type II endometrial cancer (EC) cell lines and xenografts undergo oncolysis when exposed to vaccinia virus (VV).
Copenhagen strain of VV is more potent in EC oncolysis than Wyeth strain.
A VV clinical trial in type II EC is warranted.
Acknowledgments
This work was supported by Mayo Clinic Comprehensive Cancer Center (P30CA015083), and grants from the National Institutes of Health National Cancer Institute (R01CA129193, R01CA136547, Mayo Clinic Ovarian SPORE P50CA136393), Office of Women’s Health Research Building Interdisciplinary Careers in Women’s Health (K12 HD065987) and a generous gift from Harry and Lorraine Hammerly.
The human type I cell lines were provided by William Cliby, MD, Mayo Clinic, and the type II cell lines were provided by Sean Dowdy, MD, Mayo Clinic.
Abbreviations
- DMEM
Dulbecco Modified Eagle Medium
- EC
endometrial cancer
- VV
vaccinia virus
- EEV
enveloped virus from
- VVNIS-W
Wyeth strain of VV expressing the thyroidal sodium iodide symporter
- VVNIS-C
Copenhangen strain of VV expressing the thyroidal sodium iodide symporter
- WR
Western Reserve strain of VV
- GFP
green fluorescent protein
- IFN
interferon
- hNIS
human sodium iodide symporter
- MV
measles virus
- MV-NIS
Edmonston strain MV expressing the thyroidal sodium iodide symporter
- OV
oncolytic virus
- IV
intravenous
- MOI
multiplicity of infection
- TCID50
50% tissue culture infective dose
- VSV
vesicular stomatitis virus
- HSV
herpes simplex virus
- Ad
adenovirus
- EGFR
epidermal growth factor receptor
- TK
thymidine kinase
- SCCHN
squamous cell carcinoma of the head and neck
- EC50
half maximal effective concentration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
Drs Russell, Wang, Bell and Peng, and Mayo Clinic have a financial interest in this research.
References
- 1.Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nature biotechnology. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gholami S, Chen CH, Lou E, Belin LJ, Fujisawa S, Longo VA, Chen NG, Gonen M, Zanzonico PB, Szalay AA, Fong Y. Vaccinia virus GLV-1h153 in combination with 131I shows increased efficiency in treating triple-negative breast cancer. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013 doi: 10.1096/fj.13-237222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rintoul JL, Wang J, Gammon DB, van Buuren NJ, Garson K, Jardine K, Barry M, Evans DH, Bell JC. A selectable and excisable marker system for the rapid creation of recombinant poxviruses. PloS one. 2011;6:e24643. doi: 10.1371/journal.pone.0024643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whilding LM, Archibald KM, Kulbe H, Balkwill FR, Oberg D, McNeish IA. Vaccinia Virus Induces Programmed Necrosis in Ovarian Cancer Cells. Molecular therapy : the journal of the American Society of Gene Therapy. 2013 doi: 10.1038/mt.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He S, Li P, Chen CH, Bakst RL, Chernichenko N, Yu YA, Chen N, Szalay AA, Yu Z, Fong Y, Wong RJ. Effective oncolytic vaccinia therapy for human sarcomas. The Journal of surgical research. 2012;175:e53–e60. doi: 10.1016/j.jss.2011.11.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lun X, Ruan Y, Jayanthan A, Liu DJ, Singh A, Trippett T, Bell J, Forsyth P, Johnston RN, Narendran A. Double-deleted vaccinia virus in virotherapy for refractory and metastatic pediatric solid tumors. Molecular oncology. 2013;7:944–954. doi: 10.1016/j.molonc.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen Y, Nemunaitis J. Fighting cancer with vaccinia virus: teaching new tricks to an old dog. Molecular therapy : the journal of the American Society of Gene Therapy. 2005;11:180–195. doi: 10.1016/j.ymthe.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 8.Thorne SH, Hwang TH, O'Gorman WE, Bartlett DL, Sei S, Kanji F, Brown C, Werier J, Cho JH, Lee DE, Wang Y, Bell J, Kirn DH. Rational strain selection and engineering creates a broad-spectrum, systemically effective oncolytic poxvirus, JX-963. The Journal of clinical investigation. 2007;117:3350–3358. doi: 10.1172/JCI32727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE, Roh MS, Je JE, Yoon JH, Thorne SH, Kirn D, Hwang TH. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Molecular therapy : the journal of the American Society of Gene Therapy. 2006;14:361–370. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Foloppe J, Kintz J, Futin N, Findeli A, Cordier P, Schlesinger Y, Hoffmann C, Tosch C, Balloul JM, Erbs P. Targeted delivery of a suicide gene to human colorectal tumors by a conditionally replicating vaccinia virus. Gene therapy. 2008;15:1361–1371. doi: 10.1038/gt.2008.82. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Q, Yu YA, Wang E, Chen N, Danner RL, Munson PJ, Marincola FM, Szalay AA. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer research. 2007;67:10038–10046. doi: 10.1158/0008-5472.CAN-07-0146. [DOI] [PubMed] [Google Scholar]
- 12.Dingli D, Peng KW, Harvey ME, Greipp PR, O'Connor MK, Cattaneo R, Morris JC, Russell SJ. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood. 2004;103:1641–1646. doi: 10.1182/blood-2003-07-2233. [DOI] [PubMed] [Google Scholar]
- 13.Hakkarainen T, Rajecki M, Sarparanta M, Tenhunen M, Airaksinen AJ, Desmond RA, Kairemo K, Hemminki A. Targeted radiotherapy for prostate cancer with an oncolytic adenovirus coding for human sodium iodide symporter. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5396–5403. doi: 10.1158/1078-0432.CCR-08-2571. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Nakashima H, Decklever TD, Nace RA, Russell SJ. HSV-NIS, an oncolytic herpes simplex virus type 1 encoding human sodium iodide symporter for preclinical prostate cancer radiovirotherapy. Cancer gene therapy. 2013;20:478–485. doi: 10.1038/cgt.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galanis E, Hartmann LC, Cliby WA, Long HJ, Peethambaram PP, Barrette BA, Kaur JS, Haluska PJ, Jr, Aderca I, Zollman PJ, Sloan JA, Keeney G, Atherton PJ, Podratz KC, Dowdy SC, Stanhope CR, Wilson TO, Federspiel MJ, Peng KW, Russell SJ. Phase I trial of intraperitoneal administration of an oncolytic measles virus strain engineered to express carcinoembryonic antigen for recurrent ovarian cancer. Cancer research. 2010;70:875–882. doi: 10.1158/0008-5472.CAN-09-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peng KW, TenEyck CJ, Galanis E, Kalli KR, Hartmann LC, Russell SJ. Intraperitoneal therapy of ovarian cancer using an engineered measles virus. Cancer research. 2002;62:4656–4662. [PubMed] [Google Scholar]
- 17.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: A Cancer Journal for Clinicians. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 18.Homesley HD, Filiaci V, Gibbons SK, Long HJ, Cella D, Spirtos NM, Morris RT, DeGeest K, Lee R, Montag A. A randomized phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: A Gynecologic Oncology Group study. Gynecologic oncology. 2009;112:543–552. doi: 10.1016/j.ygyno.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maggi R, Lissoni A, Spina F, Melpignano M, Zola P, Favalli G, Colombo A, Fossati R. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: results of a randomised trial. British journal of cancer. 2006;95:266–271. doi: 10.1038/sj.bjc.6603279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kokka FBE, Oram D, Gallagher C, Bryant A. Hormonal therapy in advanced or recurrent endometrial cancer. Cochrane Database Syst Rev. 2010;8:CD007926. doi: 10.1002/14651858.CD007926.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leslie KK, Sill MW, Lankes HA, Fischer EG, Godwin AK, Gray H, Schilder RJ, Walker JL, Tewari K, Hanjani P, Abulafia O, Rose PG. Lapatinib and potential prognostic value of EGFR mutations in a Gynecologic Oncology Group phase II trial of persistent or recurrent endometrial cancer. Gynecologic oncology. 2012;127:345–350. doi: 10.1016/j.ygyno.2012.07.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aghajanian C, Sill MW, Darcy KM, Greer B, McMeekin DS, Rose PG, Rotmensch J, Barnes MN, Hanjani P, Leslie KK. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2259–2265. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia AA, Blessing JA, Nolte S, Mannel RS. A phase II evaluation of weekly docetaxel in the treatment of recurrent or persistent endometrial carcinoma: a study by the Gynecologic Oncology Group. Gynecologic oncology. 2008;111:22–26. doi: 10.1016/j.ygyno.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Muggia FM, Blessing JA, Sorosky J, Reid GC. Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: a Gynecologic Oncology Group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20:2360–2364. doi: 10.1200/JCO.2002.08.171. [DOI] [PubMed] [Google Scholar]
- 25.Kim KH, Dmitriev I, O'Malley JP, Wang M, Saddekni S, You Z, Preuss MA, Harris RD, Aurigemma R, Siegal GP, Zinn KR, Curiel DT, Alvarez RD. A Phase I Clinical Trial of Ad5.SSTR/TK.RGD, a Novel Infectivity-Enhanced Bicistronic Adenovirus, in Patients with Recurrent Gynecologic Cancer. Clinical Cancer Research. 2012;18:3440–3451. doi: 10.1158/1078-0432.CCR-11-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y-P, Steele MB, Suksanpaisan L, Federspiel MJ, Russell SJ, Peng KW, Bakkum-Gamez JN. Oncolytic measles and vesicular stomatitis virotherapy for endometrial cancer. Gynecologic Oncology. doi: 10.1016/j.ygyno.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCart JA, Ward JM, Lee J, Hu Y, Alexander HR, Libutti SK, Moss B, Bartlett DL. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer research. 2001;61:8751–8757. [PubMed] [Google Scholar]
- 28.Ayeni TA, Bakkum-Gamez JN, Mariani A, McGree ME, Weaver AL, Haddock MG, Keeney GL, Long HJ, 3rd, Dowdy SC, Podratz KC. Comparative outcomes assessment of uterine grade 3 endometrioid, serous, and clear cell carcinomas. Gynecologic oncology. 2013;129:478–485. doi: 10.1016/j.ygyno.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Landrum LM, Moore KN, Myers TK, Lanneau GS, Jr, McMeekin DS, Walker JL, Gold MA. Stage IVB endometrial cancer: does applying an ovarian cancer treatment paradigm result in similar outcomes? A case-control analysis. Gynecologic oncology. 2009;112:337–341. doi: 10.1016/j.ygyno.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 30.Mader EK, Maeyama Y, Lin Y, Butler GW, Russell HM, Galanis E, Russell SJ, Dietz AB, Peng KW. Mesenchymal stem cell carriers protect oncolytic measles viruses from antibody neutralization in an orthotopic ovarian cancer therapy model. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:7246–7255. doi: 10.1158/1078-0432.CCR-09-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Putz MM, Midgley CM, Law M, Smith GL. Quantification of antibody responses against multiple antigens of the two infectious forms of Vaccinia virus provides a benchmark for smallpox vaccination. Nature medicine. 2006;12:1310–1315. doi: 10.1038/nm1457. [DOI] [PubMed] [Google Scholar]
- 32.Vanderplasschen A, Mathew E, Hollinshead M, Sim RB, Smith GL. Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7544–7549. doi: 10.1073/pnas.95.13.7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kirn DH, Thorne SH. Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nature reviews. Cancer. 2009;9:64–71. doi: 10.1038/nrc2545. [DOI] [PubMed] [Google Scholar]
- 34.Katsafanas GC, Moss B. Vaccinia virus intermediate stage transcription is complemented by Ras-GTPase-activating protein SH3 domain-binding protein (G3BP) and cytoplasmic activation/proliferation-associated protein (p137) individually or as a heterodimer. The Journal of biological chemistry. 2004;279:52210–52217. doi: 10.1074/jbc.M411033200. [DOI] [PubMed] [Google Scholar]
- 35.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 36.Hengstschlager M, Knofler M, Mullner EW, Ogris E, Wintersberger E, Wawra E. Different regulation of thymidine kinase during the cell cycle of normal versus DNA tumor virus-transformed cells. The Journal of biological chemistry. 1994;269:13836–13842. [PubMed] [Google Scholar]
- 37.Park BH, Hwang T, Liu TC, Sze DY, Kim JS, Kwon HC, Oh SY, Han SY, Yoon JH, Hong SH, Moon A, Speth K, Park C, Ahn YJ, Daneshmand M, Rhee BG, Pinedo HM, Bell JC, Kirn DH. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. The lancet oncology. 2008;9:533–542. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 38.Moerdyk-Schauwecker M, Shah NR, Murphy AM, Hastie E, Mukherjee P, Grdzelishvili VZ. Resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus: role of type I interferon signaling. Virology. 2013;436:221–234. doi: 10.1016/j.virol.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perdiguero B, Esteban M. The interferon system and vaccinia virus evasion mechanisms. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2009;29:581–598. doi: 10.1089/jir.2009.0073. [DOI] [PubMed] [Google Scholar]
- 40.Lin SF, Yu Z, Riedl C, Woo Y, Zhang Q, Yu YA, Timiryasova T, Chen N, Shah JP, Szalay AA, Fong Y, Wong RJ. Treatment of anaplastic thyroid carcinoma in vitro with a mutant vaccinia virus. Surgery. 2007;142:976–983. doi: 10.1016/j.surg.2007.09.017. discussion 976-83. [DOI] [PubMed] [Google Scholar]