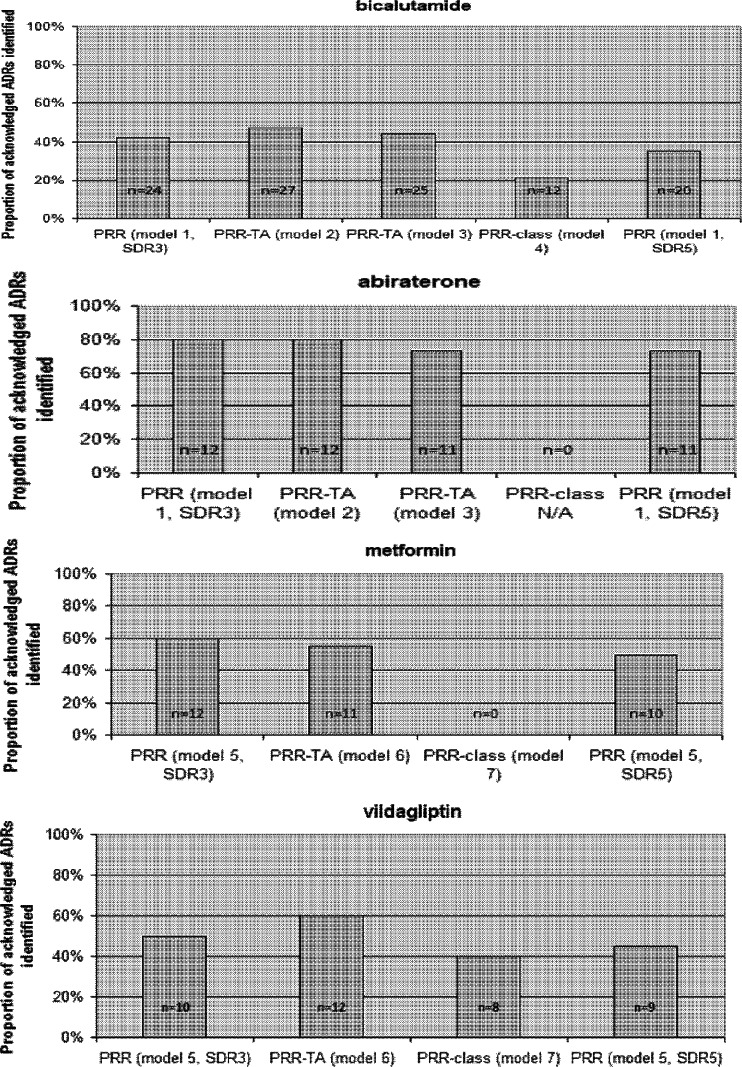
Fig. 1.
a-d The proportion of detected acknowledged ADRs, i.e., true-positive SDRs for bicalutamide (a), abiraterone (b), metformin (c), and vildagliptin (d) using from left to right for (a, b): the conventional PRR defining the SDR by a case count of ≥3 (model 1, SDR3); PRR-TA, prostate gland disease drugs (model 2, SDR3) ; PRR-TA prostate cancer drugs (model 3, SDR3); PRR-class (model 4, SDR3, not for abiraterone); and the conventional PRR defining the SDR by a case count of ≥5 (model 1, SDR5). (c, d) From left to right, the conventional PRR defining the SDR by a case count of ≥3 (SDR3); the PRR-TA(SDR3); PRR-class(SDR3); and the conventional PRR defining the SDR by a case count of ≥5 (SDR5)