Figure 6.
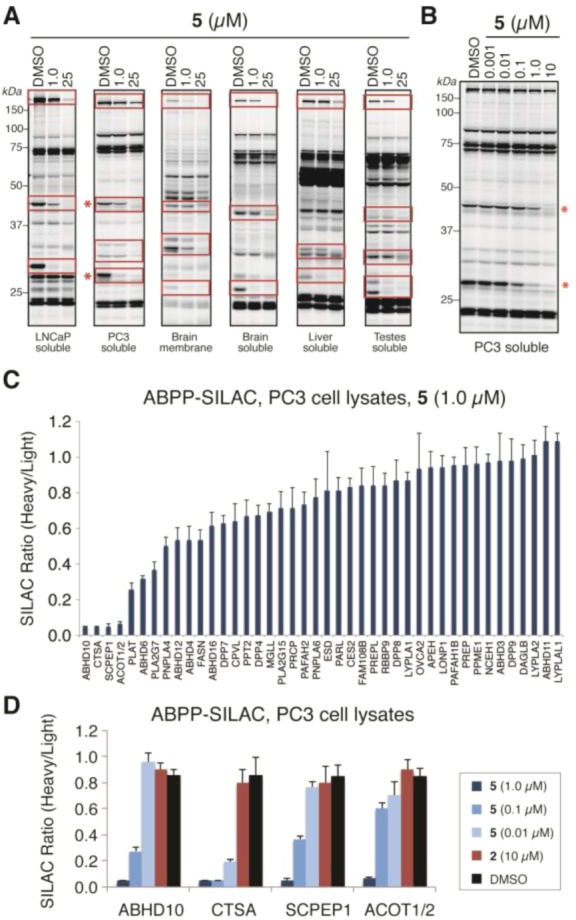
In vitro competitive ABPP of β-lactone (±)-5. (A) Gel-based ABPP of (±)-5 in various human cancer cell and mouse tissue proteomes showing inhibition of multiple serine hydrolases (highlighted in red boxes). Proteomes were treated with DMSO or (±)-5 (1.0 or 25 μM) for 30 min followed by FP-Rh (30 min). (B) Concentration-dependent inhibition of serine hydrolase activities in PC3 cell proteomes treated with DMSO or (±)-5 (0.001–10 μM), showing significant inhibition of serine hydrolase activities migrating at 30 and 45 kDa (red asterisks). (C) ABPP-SILAC analysis of (±)-5 at 1.0 μM (heavy amino acid-labeled proteome) versus DMSO (light amino acid-labeled proteome) in PC3 cell proteomes, revealing inhibition of ABHD10, CTSA, SCPEP1, and ACOT1/2. (D) ABPP-SILAC analysis of (±)-5 (1.0, 0.1, and 0.01 μM), 2 (10 μM), or DMSO (heavy) versus DMSO (light) in PC3 cell proteomes showing dose-dependent inhibition of the four primary targets of (±)-5 observed at 1.0 μM. For C and D, data are presented as the mean ± standard deviations of heavy/light ratios for multiple unique peptides from each serine hydrolase.
